Surviving change: the metabolic journey of hematopoietic stem cells
- PMID: 24768033
- PMCID: PMC4112160
- DOI: 10.1016/j.tcb.2014.04.001
Surviving change: the metabolic journey of hematopoietic stem cells
Abstract
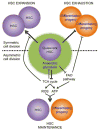
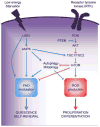
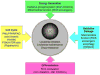
Hematopoietic stem cells (HSCs) are a rare population of somatic stem cells that maintain blood production and are uniquely wired to adapt to diverse cellular fates during the lifetime of an organism. Recent studies have highlighted a central role for metabolic plasticity in facilitating cell fate transitions and in preserving HSC functionality and survival. This review summarizes our current understanding of the metabolic programs associated with HSC quiescence, self-renewal, and lineage commitment, and highlights the mechanistic underpinnings of these changing bioenergetics programs. It also discusses the therapeutic potential of targeting metabolic drivers in the context of blood malignancies.
Keywords: blood; fatty acid oxidation; glycolysis; hematopoietic stem cells; quiescence; reactive oxygen species.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures
References
-
- Wilson A, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135(6):1118–29. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

