PTENα, a PTEN isoform translated through alternative initiation, regulates mitochondrial function and energy metabolism
- PMID: 24768297
- PMCID: PMC4097321
- DOI: 10.1016/j.cmet.2014.03.023
PTENα, a PTEN isoform translated through alternative initiation, regulates mitochondrial function and energy metabolism
Abstract
PTEN is one of the most frequently mutated genes in human cancer. It is known that PTEN has a wide range of biological functions beyond tumor suppression. Here, we report that PTENα, an N-terminally extended form of PTEN, functions in mitochondrial metabolism. Translation of PTENα is initiated from a CUG codon upstream of and in-frame with the coding region of canonical PTEN. Eukaryotic translation initiation factor 2A (eIF2A) controls PTENα translation, which requires a CUG-centered palindromic motif. We show that PTENα induces cytochrome c oxidase activity and ATP production in mitochondria. TALEN-mediated somatic deletion of PTENα impairs mitochondrial respiratory chain function. PTENα interacts with canonical PTEN to increase PINK1 protein levels and promote energy production. Our studies demonstrate the importance of eIF2A-mediated alternative translation for generation of protein diversity in eukaryotic systems and provide insights into the mechanism by which the PTEN family is involved in multiple cellular processes.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


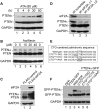
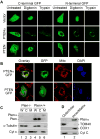



Comment in
-
Organelle dynamics: (P)TEN-ding to mitochondria.Nat Rev Mol Cell Biol. 2014 Jun;15(6):366-7. doi: 10.1038/nrm3808. Epub 2014 May 14. Nat Rev Mol Cell Biol. 2014. PMID: 24824067 No abstract available.
References
-
- Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A, Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509–1512. - PubMed
-
- Chen W, Lee PJ, Shion H, Ellor N, Gebler JC. Improving de novo sequencing of peptides using a charged tag and C-terminal digestion. Anal Chem. 2007;79:1583–1590. - PubMed
-
- Coenen MJ, van den Heuvel LP, Smeitink JA. Mitochondrial oxidative phosphorylation system assembly in man: recent achievements. Curr Opin Neurol. 2001;14:777–781. - PubMed
-
- Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. - PubMed
-
- Fang M, Shen Z, Huang S, Zhao L, Chen S, Mak TW, Wang X. The ER UDPase ENTPD5 promotes protein N-glycosylation, the Warburg effect, and proliferation in the PTEN pathway. Cell. 2010;143:711–724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

