Convergence of genes and cellular pathways dysregulated in autism spectrum disorders
- PMID: 24768552
- PMCID: PMC4067558
- DOI: 10.1016/j.ajhg.2014.03.018
Convergence of genes and cellular pathways dysregulated in autism spectrum disorders
Abstract
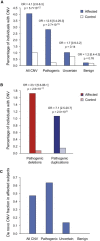
Rare copy-number variation (CNV) is an important source of risk for autism spectrum disorders (ASDs). We analyzed 2,446 ASD-affected families and confirmed an excess of genic deletions and duplications in affected versus control groups (1.41-fold, p = 1.0 × 10(-5)) and an increase in affected subjects carrying exonic pathogenic CNVs overlapping known loci associated with dominant or X-linked ASD and intellectual disability (odds ratio = 12.62, p = 2.7 × 10(-15), ∼3% of ASD subjects). Pathogenic CNVs, often showing variable expressivity, included rare de novo and inherited events at 36 loci, implicating ASD-associated genes (CHD2, HDAC4, and GDI1) previously linked to other neurodevelopmental disorders, as well as other genes such as SETD5, MIR137, and HDAC9. Consistent with hypothesized gender-specific modulators, females with ASD were more likely to have highly penetrant CNVs (p = 0.017) and were also overrepresented among subjects with fragile X syndrome protein targets (p = 0.02). Genes affected by de novo CNVs and/or loss-of-function single-nucleotide variants converged on networks related to neuronal signaling and development, synapse function, and chromatin regulation.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Constantino J.N., Todorov A., Hilton C., Law P., Zhang Y., Molloy E., Fitzgerald R., Geschwind D. Autism recurrence in half siblings: strong support for genetic mechanisms of transmission in ASD. Mol. Psychiatry. 2013;18:137–138. - PubMed
-
- Levy D., Ronemus M., Yamrom B., Lee Y.H., Leotta A., Kendall J., Marks S., Lakshmi B., Pai D., Ye K. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011;70:886–897. - PubMed
-
- Sanders S.J., Ercan-Sencicek A.G., Hus V., Luo R., Murtha M.T., Moreno-De-Luca D., Chu S.H., Moreau M.P., Gupta A.R., Thomson S.A. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70:863–885. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

