LRH1 promotes pancreatic cancer metastasis
- PMID: 24769073
- PMCID: PMC10068836
- DOI: 10.1016/j.canlet.2014.04.017
LRH1 promotes pancreatic cancer metastasis
Abstract
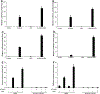
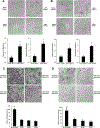
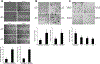
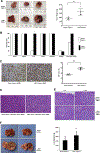
The transcriptional factor liver receptor homolog 1 (LRH1) regulates pancreatic development, and may participate in pancreatic oncogenesis through activation of growth factor signaling transduction cascades. We measured transcriptional activity of β-catenin in response to LRH1 stimulation by a Topflash reporter assay. The pancreatic cancer (PC) phenotype was then characterized by cell migration, wound healing, invasion, and sphere formation in vitro, as well as tumor formation and distant metastatic spread in vivo. We compared results between vector control and LRH1-overexpressing stable PC cell lines. In addition, tumor burden, angiogenesis, histologic characteristics, and hepatic spread were assessed in orthotopic and experimental liver metastatic murine models. Expression of downstream LRH1 related genes was evaluated by Western blot and immunohistochemistry in PC cell lines and human tumor specimens. Specific inhibition of LRH1 expression and function was accomplished by shRNAs "knockdown" experiments. It was found that LRH1 enhanced transcriptional activity of β-catenin and the expression of downstream target genes (c-Myc, MMP2/9), as well as promoted migration, wound healing, invasion, and sphere formation of PC cell lines. Specific inhibition of LRH1 by shRNAs reduced cell migration, invasion, sphere formation and expression of c-Myc and MMP2/9 target genes. Mice injected with LRH1 overexpressing stable PC cells developed tumors with increased size and exhibited striking hepatic metastatic spread. More important, LRH1 was overexpressed in PC tumors compared to adjacent normal pancreas. Our findings demonstrate that LRH1 overexpression is associated with increased PC growth and metastatic spread, indicating that LRH1-targeted therapy could inhibit tumor progression.
Keywords: Liver receptor homolog 1; MMP2; MMP9; Pancreatic cancer; c-Myc.
Copyright © 2014 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest
There is no conflict of interest among authors.
Figures



References
-
- Botrugno OA, Fayard E, Annicotte JS, Haby C, Brennan T, Wendling O, Tanaka T, Kodama T, Thomas W, Auwerx J, Schoonjans K, Synergy between LRH-1 and beta-catenin induces G1 cyclin-mediated cell proliferation, Mol. Cell 15 (2004) 499–509. - PubMed
-
- Chand AL, Herridge KA, Thompson EW, Clyne CD, The orphan nuclear receptor LRH-1 promotes breast cancer motility and invasion, Endocrine-related Cancer 17 (2010) 965–975. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

