Ocular vascular occlusive disorders: natural history of visual outcome
- PMID: 24769221
- PMCID: PMC4073304
- DOI: 10.1016/j.preteyeres.2014.04.001
Ocular vascular occlusive disorders: natural history of visual outcome
Abstract
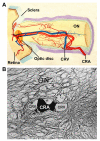
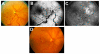
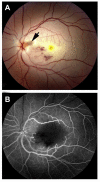
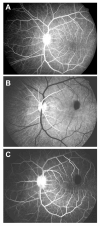
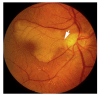
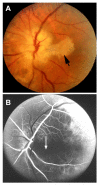
Ocular vascular occlusive disorders collectively constitute the most common cause of visual disability. Before a disease can be managed, it is essential to understand its natural history, so as to be able to assess the likely effectiveness of any intervention. I investigated natural history of visual outcome in prospective studies of 386 eyes with non-arteritic anterior ischemic optic neuropathy (NA-AION), 16 eyes with non-arteritic posterior ischemic optic neuropathy, 697 eyes with central retinal vein occlusion (CRVO), 67 eyes with hemi-CRVO (HCRVO), 216 eyes with branch retinal vein occlusion (BRVO), 260 eyes with central retinal artery occlusion (CRAO), 151 eyes with branch retinal artery occlusion (BRAO) and 61 eyes with cilioretinal artery occlusion (CLRAO). My studies have shown that every one of these disorders consists of multiple distinct clinical sub-categories with different visual findings. When an ocular vascular occlusive disorder is caused by giant cell arteritis, which is an ophthalmic emergency, it would be unethical to do a natural history study of visual outcome in them, because in this case early diagnosis and immediate, intensive high-dose steroid therapy is essential to prevent any further visual loss, not only in the involved eye but also in the fellow, normal eye. In NA-AION in eyes seen ≤2 weeks after the onset, visual acuity (VA) improved in 41% of those with VA 20/70 or worse, and visual field (VF) improved in 26% of those with moderate to severe VF defect. In non-ischemic CRVO eyes with VA 20/70 or worse, VA improved in 47% and in ischemic CRVO in 23%; moderate to severe VF defect improved in 79% in non-ischemic CRVO and in 27% in ischemic CRVO. In HCRVO, overall findings demonstrated that initial VA and VF defect and the final visual outcome were different in non-ischemic from ischemic HCRVO - much better in the former than the latter. In major BRVO, in eyes with initial VA of 20/70 or worse, VA improved in 69%, and moderate to severe VF defect improved in 52%. In macular BRVO with 20/70 or worse initial VA, it improved in 53%, and initial minimal-mild VF defect was stable or improved in 85%. In various types of CRAO there are significant differences in both initial and final VA and VF defects. In CRAO eyes seen within 7 days of onset and initial VA of counting fingers or worse, VA improved in 82% with transient non-arteritic CRAO, 67% with non-arteritic CRAO with cilioretinal artery sparing, 22% with non-arteritic CRAO. Central VF improved in 39% of transient non-arteritic CRAO, 25% of non-arteritic CRAO with cilioretinal artery sparing and 21% of non-arteritic CRAO. Peripheral VF improved in non-arteritic CRAO in 39% and in transient non-arteritic CRAO in 39%. In transient CRAO, finally peripheral VFs were normal in 93%. In non-arteritic CRAO eyes initially 22% had normal peripheral VF and in the rest it improved in 39%. Final VA of 20/40 or better was seen in 89% of permanent BRAO, and in 100% of transient BRAO and non-arteritic CLRAO. In permanent BRAO eyes, among those seen within 7 days of onset, central VF defect improved in 47% and peripheral VF in 52%, and in transient BRAO central and peripheral VFs were normal at follow-up. My studies showed that AION, CRVO, BRVO, CRAO and BRAO, each consist of multiple distinct clinical sub-categories with different visual outcome. Contrary to the prevalent impression, these studies on the natural history of visual outcome have shown that there is a statistically significant spontaneous visual improvement in each category. The factors which influence the visual outcome in various ocular vascular occlusive disorders are discussed.
Keywords: Branch retinal vein occlusion; Central retinal artery occlusion; Central retinal vein occlusion; Non-arteritic anterior ischemic optic neuropathy.
Copyright © 2014 Elsevier Ltd. All rights reserved.
Figures









References
-
- Arnold AC, Hepler RS. Natural history of nonarteritic anterior ischemic optic neuropathy. J. Neuroophthalmol. 1994;14:66–69. - PubMed
-
- Brown GC, Magargal LE. Central retinal artery obstruction and visual acuity. Ophthalmology. 1982;89:14–19. - PubMed
-
- Brown GC, Moffat K, Cruess A, Magargal LE, Goldberg RE. Cilioretinal artery obstruction. Retina. 1983;3:182–187. - PubMed
-
- Chen JC, Klein ML, Watzke RC, Handelman IL, Robertson JF. Natural course of perfused central retinal vein occlusion. Can. J. Ophthalmol. 1995;30:21–24. - PubMed
-
- Chopdar A. Hemi-central retinal vein occlusion. Pathogenesis, clinical features, natural history and incidence of dual trunk central retinal vein. Trans. Ophthalmol. Soc. U. K. 1982;102:241–248. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

