A Receptor-Like Kinase Mediates Ammonium Homeostasis and Is Important for the Polar Growth of Root Hairs in Arabidopsis
- PMID: 24769480
- PMCID: PMC4036567
- DOI: 10.1105/tpc.114.124586
A Receptor-Like Kinase Mediates Ammonium Homeostasis and Is Important for the Polar Growth of Root Hairs in Arabidopsis
Abstract
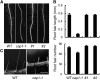
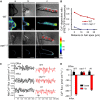
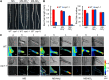
Ammonium (NH4+) is both a necessary nutrient and an important signal in plants, but can be toxic in excess. Ammonium sensing and regulatory mechanisms in plant cells have not been fully elucidated. To decipher the complex network of NH4+ signaling, we analyzed [Ca2+]cyt-associated protein kinase (CAP) genes, which encode signaling components that undergo marked changes in transcription levels in response to various stressors. We demonstrated that CAP1, a tonoplast-localized receptor-like kinase, regulates root hair tip growth by maintaining cytoplasmic Ca2+ gradients. A CAP1 knockout mutant (cap1-1) produced elevated levels of cytoplasmic NH4+. Furthermore, root hair growth of cap1-1 was inhibited on Murashige and Skoog medium, but NH4+ depletion reestablished the Ca2+ gradient necessary for normal growth. The lower net NH4+ influx across the vacuolar membrane and relatively alkaline cytosolic pH of cap1-1 root hairs implied that mutation of CAP1 increased NH4+ accumulation in the cytoplasm. Furthermore, CAP1 functionally complemented the npr1 (nitrogen permease reactivator protein) kinase yeast mutant, which is defective in high-affinity NH4+ uptake via MEP2 (methylammonium permease 2), distinguishing CAP1 as a cytosolic modulator of NH4+ levels that participates in NH4+ homeostasis-regulated root hair growth by modulating tip-focused cytoplasmic Ca2+ gradients.
© 2014 American Society of Plant Biologists. All rights reserved.
Figures




References
-
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 - PubMed
-
- Babourina O., Voltchanskii K., McGann B., Newman I., Rengel Z. (2007). Nitrate supply affects ammonium transport in canola roots. J. Exp. Bot. 58: 651–658 - PubMed
-
- Bei Q., Luan S. (1998). Functional expression and characterization of a plant K+ channel gene in a plant cell model. Plant J. 13: 857–865 - PubMed
-
- Bernhardt C., Lee M.M., Gonzalez A., Zhang F., Lloyd A., Schiefelbein J. (2003). The bHLH genes GLABRA3 (GL3) and ENHANCER OF GLABRA3 (EGL3) specify epidermal cell fate in the Arabidopsis root. Development 130: 6431–6439 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

