Electronic versus paper-based assessment of health-related quality of life specific to HIV disease: reliability study of the PROQOL-HIV questionnaire
- PMID: 24769643
- PMCID: PMC4019778
- DOI: 10.2196/jmir.3330
Electronic versus paper-based assessment of health-related quality of life specific to HIV disease: reliability study of the PROQOL-HIV questionnaire
Abstract
Background: Electronic patient-reported outcomes (PRO) provide quick and usually reliable assessments of patients' health-related quality of life (HRQL).
Objective: An electronic version of the Patient-Reported Outcomes Quality of Life-human immunodeficiency virus (PROQOL-HIV) questionnaire was developed, and its face validity and reliability were assessed using standard psychometric methods.
Methods: A sample of 80 French outpatients (66% male, 52/79; mean age 46.7 years, SD 10.9) were recruited. Paper-based and electronic questionnaires were completed in a randomized crossover design (2-7 day interval). Biomedical data were collected. Questionnaire version and order effects were tested on full-scale scores in a 2-way ANOVA with patients as random effects. Test-retest reliability was evaluated using Pearson and intraclass correlation coefficients (ICC, with 95% confidence interval) for each dimension. Usability testing was carried out from patients' survey reports, specifically, general satisfaction, ease of completion, quality and clarity of user interface, and motivation to participate in follow-up PROQOL-HIV electronic assessments.
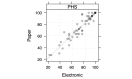
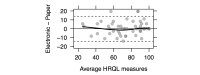
Results: Questionnaire version and administration order effects (N=59 complete cases) were not significant at the 5% level, and no interaction was found between these 2 factors (P=.94). Reliability indexes were acceptable, with Pearson correlations greater than .7 and ICCs ranging from .708 to .939; scores were not statistically different between the two versions. A total of 63 (79%) complete patients' survey reports were available, and 55% of patients (30/55) reported being satisfied and interested in electronic assessment of their HRQL in clinical follow-up. Individual ratings of PROQOL-HIV user interface (85%-100% of positive responses) confirmed user interface clarity and usability.
Conclusions: The electronic PROQOL-HIV introduces minor modifications to the original paper-based version, following International Society for Pharmacoeconomics and Outcomes Research (ISPOR) ePRO Task Force guidelines, and shows good reliability and face validity. Patients can complete the computerized PROQOL-HIV questionnaire and the scores from the paper or electronic versions share comparable accuracy and interpretation.
Keywords: HIV; electronic records; patient-reported outcomes; quality of life; reliability.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
References
-
- Reiser SJ. The clinical record in medicine. Part 1: Learning from cases. Ann Intern Med. 1991 May 15;114(10):902–7. - PubMed
-
- Tang PC, LaRosa MP, Gorden SM. Use of computer-based records, completeness of documentation, and appropriateness of documented clinical decisions. J Am Med Inform Assoc. 1999 Jun;6(3):245–51. http://jamia.bmj.com/cgi/pmidlookup?view=long&pmid=10332657 - PMC - PubMed
-
- US Department of Health and Human Services. Food and Drug Administration . Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Silver Spring, MD: Center for Drug Evaluation and Research, Food and Drug Administration; 2009. [2014-02-12]. 6NKQY89wp http://www.fda.gov/downloads/Drugs/Guidances/UCM193282.pdf. - PubMed
-
- Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient non-compliance with paper diaries. BMJ. 2002 May 18;324(7347):1193–4. http://europepmc.org/abstract/MED/12016186 - PMC - PubMed
-
- Deshpande PR, Rajan S, Sudeepthi BL, Abdul Nazir CP. Patient-reported outcomes: A new era in clinical research. Perspect Clin Res. 2011 Oct;2(4):137–44. doi: 10.4103/2229-3485.86879. http://www.picronline.org/article.asp?issn=2229-3485;year=2011;volume=2;... - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

