Programmed cell death during neuronal development: the sympathetic neuron model
- PMID: 24769728
- PMCID: PMC4207485
- DOI: 10.1038/cdd.2014.47
Programmed cell death during neuronal development: the sympathetic neuron model
Abstract
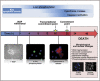
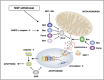
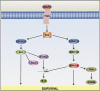
Developing sympathetic neurons of the superior cervical ganglion are one of the best studied models of neuronal apoptosis. These cells require nerve growth factor (NGF) for survival at the time that they innervate their final target tissues during late embryonic and early postnatal development. In the absence of NGF, developing sympathetic neurons die by apoptosis in a transcription-dependent manner. Molecular studies of sympathetic neuron apoptosis began in the 1980s. We now know that NGF withdrawal activates the mitochondrial (intrinsic) pathway of apoptosis in sympathetic neurons cultured in vitro, and the roles of caspases, Bcl-2 (B-cell CLL/lymphoma 2) family proteins and XIAP (X-linked inhibitor of apoptosis protein) have been extensively studied. Importantly, a considerable amount has also been learned about the intracellular signalling pathways and transcription factors that regulate programmed cell death in sympathetic neurons. In this article, we review the key papers published in the past few years, covering all aspects of apoptosis regulation in sympathetic neurons and focusing, in particular, on how signalling pathways and transcription factors regulate the cell death programme. We make some comparisons with other models of neuronal apoptosis and describe possible future directions for the field.
Figures


References
-
- Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. - PubMed
-
- Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. - PubMed
-
- Buss RR, Sun W, Oppenheim RW. Adaptive roles of programmed cell death during nervous system development. Annu Rev Neurosci. 2006;29:1–35. - PubMed
-
- Wright LL, Cunningham TJ, Smolen AJ. Developmental neuron death in the rat superior cervical sympathetic ganglion: cell counts and ultrastructure. J Neurocytol. 1983;12:727–738. - PubMed
-
- Glebova NO, Ginty DD. Growth and survival signals controlling sympathetic nervous system development. Annu Rev Neurosci. 2005;28:191–222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

