Lipopolysaccharide (LPS) inhibits steroid production in theca cells of bovine follicles in vitro: distinct effect of LPS on theca cell function in pre- and post-selection follicles
- PMID: 24769841
- PMCID: PMC4139502
- DOI: 10.1262/jrd.2013-124
Lipopolysaccharide (LPS) inhibits steroid production in theca cells of bovine follicles in vitro: distinct effect of LPS on theca cell function in pre- and post-selection follicles
Abstract
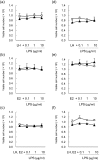
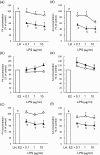
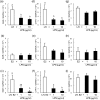
In postpartum dairy cows, lipopolysaccharide (LPS) derived from gram-negative bacteria such as Escherichia coli causes uterine inflammation and leads to ovarian dysfunction. The aim of this study was to determine the effect of LPS on steroid production in bovine theca cells at different stages of follicular development. Theca cells isolated from pre- and post-selection follicles (PRFs, <8.5 mm in diameter, and POFs, >8.5 mm in diameter, respectively) of bovine ovaries were exposed to LPS under luteinizing hormone (LH) conditions, estradiol (E2) conditions or both conditions in vitro. Bovine theca cells expressed the LPS receptor gene complex: Toll-like receptor 4 (TLR4), CD14 and MD2. LPS suppressed progesterone (P4) and androstenedione (A4) production with downregulation of steroidogenic enzyme transcripts when theca cells were stimulated with LH. By contrast, LPS did not affect P4 or A4 production when theca cells were stimulated with E2. P4 and A4 production in theca cells from PRFs was suppressed by LPS as early as at 48 h of culture, whereas the effect of LPS on theca cells from POFs was observed at 96 h of culture. The results demonstrate that LPS inhibits steroid production in theca cells under LH conditions. Moreover, theca cells from POFs showed a slower response to LPS compared with that of theca cells from PRFs, which might imply a distinct effect of LPS on follicles at different developmental stages. These findings suggest a possible mechanism of ovarian dysfunction and subsequent infertility in cows with endometritis.
Figures



Similar articles
-
Peptidoglycan inhibits progesterone and androstenedione production in bovine ovarian theca cells.Toxicol In Vitro. 2014 Aug;28(5):961-7. doi: 10.1016/j.tiv.2014.04.005. Epub 2014 Apr 13. Toxicol In Vitro. 2014. PMID: 24727680
-
Androgen production in response to LH is impaired in theca cells from nonovulatory dominant follicles in early-postpartum dairy cows.Domest Anim Endocrinol. 2020 Apr;71:106385. doi: 10.1016/j.domaniend.2019.106385. Epub 2019 Aug 8. Domest Anim Endocrinol. 2020. PMID: 31726391
-
Oxytocin inhibits LH-stimulated production of androstenedione by bovine theca cells.Mol Cell Endocrinol. 2002 Feb 25;188(1-2):151-9. doi: 10.1016/s0303-7207(01)00727-4. Mol Cell Endocrinol. 2002. PMID: 11911954
-
Effects of inhibin-related peptides and oestradiol on androstenedione and progesterone secretion by bovine theca cells in vitro.J Endocrinol. 1995 Jun;145(3):491-500. doi: 10.1677/joe.0.1450491. J Endocrinol. 1995. PMID: 7636433
-
Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle.Biol Reprod. 2009 Dec;81(6):1025-32. doi: 10.1095/biolreprod.109.077370. Epub 2009 May 13. Biol Reprod. 2009. PMID: 19439727 Free PMC article. Review.
Cited by
-
Molecular Mechanisms of Lipopolysaccharide (LPS) Induced Inflammation in an Immortalized Ovine Luteal Endothelial Cell Line (OLENDO).Vet Sci. 2022 Feb 24;9(3):99. doi: 10.3390/vetsci9030099. Vet Sci. 2022. PMID: 35324827 Free PMC article.
-
Lipopolysaccharide-binding protein in follicular fluid is associated with the follicular inflammatory status and granulosa cell steroidogenesis in dairy cows.J Reprod Dev. 2024 Jun 1;70(3):169-176. doi: 10.1262/jrd.2023-104. Epub 2024 Apr 19. J Reprod Dev. 2024. PMID: 38644218 Free PMC article.
-
Potential mechanisms and therapeutic strategies for LPS-associated female fertility decline.J Assist Reprod Genet. 2024 Oct;41(10):2739-2758. doi: 10.1007/s10815-024-03226-2. Epub 2024 Aug 21. J Assist Reprod Genet. 2024. PMID: 39167249 Review.
-
Lipopolysaccharide affects metabolic processes and energy homeostasis in the corpus luteum.Front Mol Biosci. 2025 Jan 7;11:1523098. doi: 10.3389/fmolb.2024.1523098. eCollection 2024. Front Mol Biosci. 2025. PMID: 39845899 Free PMC article.
-
Lipopolysaccharide alters CEBPβ signaling and reduces estradiol production in bovine granulosa cells.CABI Agric Biosci. 2022;3(1):66. doi: 10.1186/s43170-022-00133-3. Epub 2022 Oct 22. CABI Agric Biosci. 2022. PMID: 37576606 Free PMC article.
References
-
- Borsberry S, Dobson H. Periparturient diseases and their effect on reproductive performance in five dairy herds. Vet Rec 1989; 124: 217–219 - PubMed
-
- LeBlanc SJ, Duffield TF, Leslie KE, Bateman KG, Keefe GP, Walton JS, Johnson WH. Defining and diagnosing postpartum clinical endometritis and its impact on reproductive performance in dairy cows. J Dairy Sci 2002; 85: 2223–2236 - PubMed
-
- Sheldon IM, Noakes DE, Rycroft AN, Pfeiffer DU, Dobson H. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction 2002; 123: 837–845 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

