P2Y13 receptor regulates HDL metabolism and atherosclerosis in vivo
- PMID: 24769858
- PMCID: PMC4000210
- DOI: 10.1371/journal.pone.0095807
P2Y13 receptor regulates HDL metabolism and atherosclerosis in vivo
Abstract
High-density lipoprotein (HDL) is known to protect against atherosclerosis by promoting the reverse cholesterol transport. A new pathway for the regulation of HDL-cholesterol (HDL-c) removal involving F1-ATPase and P2Y13 receptor (P2Y13R) was described in vitro, and recently in mice. However, the physiological role of F1-ATPase/P2Y13R pathway in the modulation of vascular pathology i.e. in the development of atherosclerotic plaques is still unknown. We designed a specific novel agonist (CT1007900) of the P2Y13R that caused stimulation of bile acid secretion associated with an increased uptake of HDL-c in the liver after single dosing in mice. Repeated dose administration in mice, for 2 weeks, stimulated the apoA-I synthesis and formation of small HDL particles. Plasma samples from the agonist-treated mice had high efflux capacity for mobilization of cholesterol in vitro compared to placebo group. In apoE-/- mice this agonist induced a decrease of atherosclerotic plaques in aortas and carotids. The specificity of P2Y13R pathway in those mice was assessed using adenovirus encoding P2Y13R-shRNA. These results demonstrate that P2Y13R plays a pivotal role in the HDL metabolism and could also be a useful therapeutic agent to decrease atherosclerosis. In this study, the up-regulation of HDL-c metabolism via activation of the P2Y13R using agonists could promote reverse cholesterol transport and promote inhibition of atherosclerosis progression in mice.
Conflict of interest statement
Figures
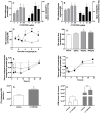
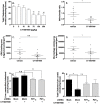
 ) and compared to vehicle treated animals (○). Values in pre-dose groups for plasma cholesterol vary from 0.85 to 0.95 g/L, and 1.1 to 1.3 g/L for plasma apoA-I. Panel G, C57Bl/6J mice (n = 5) were intravenously injected with [3H]-cholesterol-labelled mouse HDL (10 µCi/mouse) and CT1007900 (10 nmole/kg or 4 µg/kg). Radioactivity present in the liver was determined 2 hours later. **p<0.01. Panel H, C57Bl/6J mice (n = 5) were dosed (single dosing) with CT1007900 (100 µg/kg) and intravenously injected with [3H]-cholesterol-labelled mouse HDL (10 µCi/mouse). Feces from individual mouse were collected for 6 h and extracted for cholesterol (empty bars) and bile acid content (grey bars) and the radioactivity was determined by scintillation counting. *p<0.05.
) and compared to vehicle treated animals (○). Values in pre-dose groups for plasma cholesterol vary from 0.85 to 0.95 g/L, and 1.1 to 1.3 g/L for plasma apoA-I. Panel G, C57Bl/6J mice (n = 5) were intravenously injected with [3H]-cholesterol-labelled mouse HDL (10 µCi/mouse) and CT1007900 (10 nmole/kg or 4 µg/kg). Radioactivity present in the liver was determined 2 hours later. **p<0.01. Panel H, C57Bl/6J mice (n = 5) were dosed (single dosing) with CT1007900 (100 µg/kg) and intravenously injected with [3H]-cholesterol-labelled mouse HDL (10 µCi/mouse). Feces from individual mouse were collected for 6 h and extracted for cholesterol (empty bars) and bile acid content (grey bars) and the radioactivity was determined by scintillation counting. *p<0.05.

References
-
- Lee JM, Choudhury RP (2010) Atherosclerosis regression and high-density lipoproteins. Expert Rev Cardiovasc Ther 8: 1325–1334. - PubMed
-
- Brown WV, Brewer HB, Rader DJ, Schaefer EJ (2010) HDL as a treatment target. J Clin Lipidol 4: 5–16. - PubMed
-
- Duffy D, Rader DJ (2009) Update on strategies to increase HDL quantity and function. Nat Rev Cardiol 6: 455–463. - PubMed
-
- Katsiki N, Nikolic D, Montalto G, Banach M, Mikhailidis DP, et al. (2013) The role of fibrate treatment in dyslipidemia: an overview. Curr Pharm Des 19: 3124–3131. - PubMed
-
- Kamanna VS, Kashyap ML (2008) Mechanism of action of niacin. The American journal of cardiology 101: 20B–26B. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

