Inefficient translocation of preproinsulin contributes to pancreatic β cell failure and late-onset diabetes
- PMID: 24770419
- PMCID: PMC4047398
- DOI: 10.1074/jbc.M114.562355
Inefficient translocation of preproinsulin contributes to pancreatic β cell failure and late-onset diabetes
Abstract
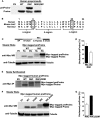
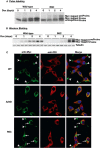
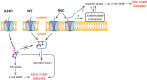
Among the defects in the early events of insulin biosynthesis, proinsulin misfolding and endoplasmic reticulum (ER) stress have drawn increasing attention as causes of β cell failure. However, no studies have yet addressed potential defects at the cytosolic entry point of preproinsulin into the secretory pathway. Here, we provide the first evidence that inefficient translocation of preproinsulin (caused by loss of a positive charge in the n region of its signal sequence) contributes to β cell failure and diabetes. Specifically, we find that, after targeting to the ER membrane, preproinsulin signal peptide (SP) mutants associated with autosomal dominant late-onset diabetes fail to be fully translocated across the ER membrane. The newly synthesized, untranslocated preproinsulin remains strongly associated with the ER membrane, exposing its proinsulin moiety to the cytosol. Rather than accumulating in the ER and inducing ER stress, untranslocated preproinsulin accumulates in a juxtanuclear compartment distinct from the Golgi complex, induces the expression of heat shock protein 70 (HSP70), and promotes β cell death. Restoring an N-terminal positive charge to the mutant preproinsulin SP significantly improves the translocation defect. These findings not only reveal a novel molecular pathogenesis of β cell failure and diabetes but also provide the first evidence of the physiological and pathological significance of the SP n region positive charge of secretory proteins.
Keywords: Cytosolic Protein Accumulation; Diabetes; Insulin Synthesis; Mutant; Preproinsulin; Proinsulin; Protein Translocation; β Cell.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Dodson G, Steiner D. (1998) The role of assembly in insulin's biosynthesis. Curr. Opin. Struct. Biol. 8, 189–194 - PubMed
-
- Okun M. M., Shields D. (1992) Translocation of preproinsulin across the endoplasmic reticulum membrane. The relationship between nascent polypeptide size and extent of signal recognition particle-mediated inhibition of protein synthesis. J. Biol. Chem. 267, 11476–11482 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

