Lumbar intervertebral disc puncture under C-arm fluoroscopy: a new rat model of lumbar intervertebral disc degeneration
- PMID: 24770648
- PMCID: PMC4160990
- DOI: 10.1538/expanim.63.227
Lumbar intervertebral disc puncture under C-arm fluoroscopy: a new rat model of lumbar intervertebral disc degeneration
Abstract
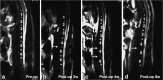
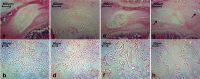
To establish a minimally invasive rat model of lumbar intervertebral disc degeneration (IDD) to better understand the pathophysiology of the human condition. The annulus fibrosus of lumbar level 4-5 (L4-5) and L5-6 discs were punctured by 27-gauge needles using the posterior approach under C-arm fluoroscopic guidance. Magnetic resonance imaging (MRI), histological examination by hematoxylin and eosin (H&E) staining, and reverse transcription polymerase chain reaction (RT-PCR) were performed at baseline and 2, 4, and 8 weeks after disc puncture surgery to determine the degree of degeneration. All sixty discs (thirty rats) were punctured successfully. Only two of thirty rats subjected to the procedure exhibited immediate neurological symptoms. The MRI results indicated a gradual increase in Pfirrmann grade from 4 to 8 weeks post-surgery (P<0.05), and H&E staining demonstrated a parallel increase in histological grade (P<0.05). Expression levels of aggrecan, type II collagen (Col2), and Sox9 mRNAs, which encode disc components, decreased gradually post-surgery. In contrast, mRNA expression of type I collagen (Col1), an indicator of fibrosis, increased (P<0.05). The procedure of annular puncture using a 27-gauge needle under C-arm fluoroscopic guidance had a high success rate. Histological, MRI, and RT-PCR results revealed that the rat model of disc degeneration is a progressive pathological process that is similar to human IDD.
Figures



Similar articles
-
Comparative expression of matrix-associated genes and inflammatory cytokines-associated genes according to disc degeneration: analysis of living human nucleus pulposus.J Spinal Disord Tech. 2011 Aug;24(6):352-7. doi: 10.1097/BSD.0b013e3181fee4df. J Spinal Disord Tech. 2011. PMID: 21150669
-
Effect of Static Compression Loads on Intervertebral Disc: An in Vivo Bent Rat Tail Model.Orthop Surg. 2018 May;10(2):134-143. doi: 10.1111/os.12377. Epub 2018 May 16. Orthop Surg. 2018. PMID: 29770581 Free PMC article.
-
Lovastatin prevents discography-associated degeneration and maintains the functional morphology of intervertebral discs.Spine J. 2014 Oct 1;14(10):2459-66. doi: 10.1016/j.spinee.2014.03.050. Epub 2014 Apr 5. Spine J. 2014. PMID: 24713605
-
Minimal invasive annulotomy for induction of disc degeneration and implantation of poly (lactic-co-glycolic acid) (PLGA) plugs for annular repair in a rabbit model.Eur J Med Res. 2016 Feb 29;21:7. doi: 10.1186/s40001-016-0202-4. Eur J Med Res. 2016. PMID: 26924131 Free PMC article.
-
The effect of bone morphogenetic protein-2 on rat intervertebral disc cells in vitro.Spine (Phila Pa 1976). 2003 Aug 15;28(16):1773-80. doi: 10.1097/01.BRS.0000083204.44190.34. Spine (Phila Pa 1976). 2003. PMID: 12923462
Cited by
-
Advanced Glycation End Product Inhibitor Pyridoxamine Attenuates IVD Degeneration in Type 2 Diabetic Rats.Int J Mol Sci. 2020 Dec 19;21(24):9709. doi: 10.3390/ijms21249709. Int J Mol Sci. 2020. PMID: 33352698 Free PMC article.
-
Diffusion kurtosis imaging provides quantitative assessment of the microstructure changes of disc degeneration: an in vivo experimental study.Eur Spine J. 2019 May;28(5):1005-1013. doi: 10.1007/s00586-019-05924-3. Epub 2019 Feb 18. Eur Spine J. 2019. PMID: 30778770
-
Optimization of a rat lumbar IVD degeneration model for low back pain.JOR Spine. 2020 Jun 22;3(2):e1092. doi: 10.1002/jsp2.1092. eCollection 2020 Jun. JOR Spine. 2020. PMID: 32613167 Free PMC article.
-
Time-Course Investigation of Intervertebral Disc Degeneration Induced by Different Sizes of Needle Punctures in Rat Tail Disc.Med Sci Monit. 2018 Sep 14;24:6456-6465. doi: 10.12659/MSM.910636. Med Sci Monit. 2018. PMID: 30216335 Free PMC article.
-
Omega-3 Fatty Acid Supplementation Reduces Intervertebral Disc Degeneration.Med Sci Monit. 2019 Dec 14;25:9531-9537. doi: 10.12659/MSM.918649. Med Sci Monit. 2019. PMID: 31836696 Free PMC article.
References
-
- An H.S., Takegami K., Kamada H., Nguyen C.M., Thonar E.J., Singh K., Andersson G.B., Masuda K.2005. Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine 30: 25–31, discussion 31–32. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

