BTEB2 prevents neuronal apoptosis via promoting bad phosphorylation in rat intracerebral hemorrhage model
- PMID: 24770868
- PMCID: PMC4289975
- DOI: 10.1007/s12031-014-0305-8
BTEB2 prevents neuronal apoptosis via promoting bad phosphorylation in rat intracerebral hemorrhage model
Abstract
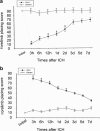
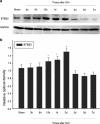
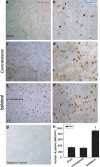
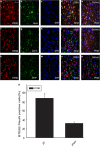
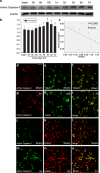
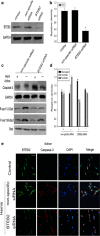
Krüppel-like zinc-finger transcription factor 5 (KLF5), known as BTEB2 or IKLF, has several biological functions that involve cell proliferation, development and apoptosis. Previous studies demonstrated that BTEB2 had anti-apoptotic effect in multiple diseases such as esophageal cancer and non-small cell lung cancers (NSCLCs). However, the distribution and function of BTEB2 in CNS diseases remain unknown. In this study, we show that BTEB2 down-regulates neuronal apoptosis during pathophysiological processes of intracerebral hemorrhage (ICH). A rat ICH model was established by behavioral tests. Western blot and immunohistochemistry revealed a remarkable up-regulation of BTEB2 expression surrounding the hematoma after ICH. Double-labeled immunofluorescence showed BTEB2 was mostly co-localized with neurons, rarely with activated astrocytes and microglia. Furthermore, we detected that neuronal apoptosis marker active caspase-3 had co-localizations with BTEB2. In addition, KLF5 knockdown in vitro specifically resulted in increasing neuronal apoptosis coupled with reduced Bad phosphorylation at both ser112 and ser136 residues. All our findings suggested that BTEB2 down-regulated neuronal apoptosis via promoting Bad phosphorylation after ICH.
Figures
Similar articles
-
Upregulated expression of SSTR1 is involved in neuronal apoptosis and is coupled to the reduction of bcl-2 following intracerebral hemorrhage in adult rats.Cell Mol Neurobiol. 2014 Oct;34(7):951-61. doi: 10.1007/s10571-014-0081-6. Epub 2014 Jul 18. Cell Mol Neurobiol. 2014. PMID: 25035058 Free PMC article.
-
Upregulated Expression of SSTR3 is Involved in Neuronal Apoptosis After Intracerebral Hemorrhage in Adult Rats.Cell Mol Neurobiol. 2017 Nov;37(8):1407-1416. doi: 10.1007/s10571-017-0471-7. Epub 2017 Feb 7. Cell Mol Neurobiol. 2017. PMID: 28176050 Free PMC article.
-
Up-regulation of Glis2 involves in neuronal apoptosis after intracerebral hemorrhage in adult rats.Cell Mol Neurobiol. 2015 Apr;35(3):345-354. doi: 10.1007/s10571-014-0130-1. Epub 2014 Nov 5. Cell Mol Neurobiol. 2015. PMID: 25370802 Free PMC article.
-
CDK5 contributes to neuronal apoptosis via promoting MEF2D phosphorylation in rat model of intracerebral hemorrhage.J Mol Neurosci. 2015 May;56(1):48-59. doi: 10.1007/s12031-014-0466-5. Epub 2014 Nov 23. J Mol Neurosci. 2015. PMID: 25417143
-
Up-regulation of Vps4A promotes neuronal apoptosis after intracerebral hemorrhage in adult rats.Metab Brain Dis. 2017 Apr;32(2):565-575. doi: 10.1007/s11011-016-9943-6. Epub 2017 Jan 7. Metab Brain Dis. 2017. PMID: 28064406
Cited by
-
Kruppel-like Pluripotency Factors as Modulators of Cancer Cell Therapeutic Responses.Cancer Res. 2016 Apr 1;76(7):1677-82. doi: 10.1158/0008-5472.CAN-15-1806. Epub 2016 Mar 10. Cancer Res. 2016. PMID: 26964625 Free PMC article. Review.
-
Association between serum levels of caspase-cleaved cytokeratin-18 and early mortality in patients with severe spontaneous intracerebral hemorrhage.BMC Neurosci. 2018 Apr 16;19(1):23. doi: 10.1186/s12868-018-0424-1. BMC Neurosci. 2018. PMID: 29661155 Free PMC article.
-
New insights into KLFs and SOXs in cancer pathogenesis, stemness, and therapy.Semin Cancer Biol. 2023 May;90:29-44. doi: 10.1016/j.semcancer.2023.02.003. Epub 2023 Feb 15. Semin Cancer Biol. 2023. PMID: 36806560 Free PMC article. Review.
-
Upregulated expression of SSTR1 is involved in neuronal apoptosis and is coupled to the reduction of bcl-2 following intracerebral hemorrhage in adult rats.Cell Mol Neurobiol. 2014 Oct;34(7):951-61. doi: 10.1007/s10571-014-0081-6. Epub 2014 Jul 18. Cell Mol Neurobiol. 2014. PMID: 25035058 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

