Uterine epithelial cell proliferation and endometrial hyperplasia: evidence from a mouse model
- PMID: 24770950
- PMCID: PMC4106634
- DOI: 10.1093/molehr/gau033
Uterine epithelial cell proliferation and endometrial hyperplasia: evidence from a mouse model
Abstract
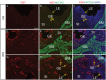
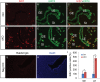
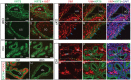
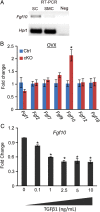
In the uterus, epithelial cell proliferation changes during the estrous cycle and pregnancy. Uncontrolled epithelial cell proliferation results in implantation failure and/or cancer development. Transforming growth factor-β (TGF-β) signaling is a fundamental regulator of diverse biological processes and is indispensable for multiple reproductive functions. However, the in vivo role of TGF-β signaling in uterine epithelial cells remains poorly defined. We have shown that in the uterus, conditional deletion of the Type 1 receptor for TGF-β (Tgfbr1) using anti-Müllerian hormone receptor type 2 (Amhr2) Cre leads to myometrial defects. Here, we describe enhanced epithelial cell proliferation by immunostaining of Ki67 in the uteri of these mice. The aberration culminated in endometrial hyperplasia in aged females. To exclude the potential influence of ovarian steroid hormones, the proliferative status of uterine epithelial cells was assessed following ovariectomy. Increased uterine epithelial cell proliferation was also revealed in ovariectomized Tgfbr1 Amhr2-Cre conditional knockout mice. We further demonstrated that transcript levels for fibroblast growth factor 10 (Fgf10) were markedly up-regulated in Tgfbr1 Amhr2-Cre conditional knockout uteri. Consistently, treatment of primary uterine stromal cells with TGF-β1 significantly reduced Fgf10 mRNA expression. Thus, our findings suggest a potential involvement of TGFBR1-mediated signaling in the regulation of uterine epithelial cell proliferation, and provide genetic evidence supporting the role of uterine epithelial cell proliferation in the pathogenesis of endometrial hyperplasia.
Keywords: endometrial hyperplasia; epithelial cell; proliferation; transforming growth factor β; uterus.
© The Author 2014. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



Similar articles
-
The uterine epithelial loss of Pten is inefficient to induce endometrial cancer with intact stromal Pten.PLoS Genet. 2018 Aug 24;14(8):e1007630. doi: 10.1371/journal.pgen.1007630. eCollection 2018 Aug. PLoS Genet. 2018. PMID: 30142194 Free PMC article.
-
Glandular defects in the mouse uterus with sustained activation of TGF-beta signaling is associated with altered differentiation of endometrial stromal cells and formation of stromal compartment.PLoS One. 2018 Dec 14;13(12):e0209417. doi: 10.1371/journal.pone.0209417. eCollection 2018. PLoS One. 2018. PMID: 30550590 Free PMC article.
-
Transforming growth factor β receptor type 1 is essential for female reproductive tract integrity and function.PLoS Genet. 2011 Oct;7(10):e1002320. doi: 10.1371/journal.pgen.1002320. Epub 2011 Oct 20. PLoS Genet. 2011. PMID: 22028666 Free PMC article.
-
Critical tumor suppressor function mediated by epithelial Mig-6 in endometrial cancer.Cancer Res. 2013 Aug 15;73(16):5090-9. doi: 10.1158/0008-5472.CAN-13-0241. Epub 2013 Jun 27. Cancer Res. 2013. PMID: 23811943 Free PMC article.
-
Mouse models of uterine corpus tumors: clinical significance and utility.Front Biosci (Elite Ed). 2010 Jun 1;2(3):882-905. doi: 10.2741/e149. Front Biosci (Elite Ed). 2010. PMID: 20515761 Review.
Cited by
-
The uterine epithelial loss of Pten is inefficient to induce endometrial cancer with intact stromal Pten.PLoS Genet. 2018 Aug 24;14(8):e1007630. doi: 10.1371/journal.pgen.1007630. eCollection 2018 Aug. PLoS Genet. 2018. PMID: 30142194 Free PMC article.
-
Transforming growth factor beta signaling and decidual integrity in mice†.Biol Reprod. 2020 Dec 1;103(6):1186-1198. doi: 10.1093/biolre/ioaa155. Biol Reprod. 2020. PMID: 32902612 Free PMC article.
-
Crosstalk between SOX2 and cytokine signaling in endometrial carcinoma.Sci Rep. 2018 Dec 3;8(1):17550. doi: 10.1038/s41598-018-35592-0. Sci Rep. 2018. PMID: 30510261 Free PMC article.
-
Molecular characterization of Wdr13 knockout female mice uteri: a model for human endometrial hyperplasia.Sci Rep. 2020 Sep 3;10(1):14621. doi: 10.1038/s41598-020-70773-w. Sci Rep. 2020. PMID: 32883989 Free PMC article.
-
A Smad3-PTEN regulatory loop controls proliferation and apoptotic responses to TGF-β in mouse endometrium.Cell Death Differ. 2017 Aug;24(8):1443-1458. doi: 10.1038/cdd.2017.73. Epub 2017 May 19. Cell Death Differ. 2017. PMID: 28524854 Free PMC article.
References
-
- Arango NA, Kobayashi A, Wang Y, Jamin SP, Lee HH, Orvis GD, Behringer RR. A mesenchymal perspective of Mullerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev. 2008;75:1154–1162. - PubMed
-
- Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. - PubMed
-
- Bazer FW. Uterine adenogenesis and pregnancy: multiple roles for foxa2 in mice. Biol Reprod. 2010;83:319–321. - PubMed
-
- Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-β superfamily. Endocr Rev. 2002;23:787–823. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

