Non-enzymatic glycolysis and pentose phosphate pathway-like reactions in a plausible Archean ocean
- PMID: 24771084
- PMCID: PMC4023395
- DOI: 10.1002/msb.20145228
Non-enzymatic glycolysis and pentose phosphate pathway-like reactions in a plausible Archean ocean
Abstract
The reaction sequences of central metabolism, glycolysis and the pentose phosphate pathway provide essential precursors for nucleic acids, amino acids and lipids. However, their evolutionary origins are not yet understood. Here, we provide evidence that their structure could have been fundamentally shaped by the general chemical environments in earth's earliest oceans. We reconstructed potential scenarios for oceans of the prebiotic Archean based on the composition of early sediments. We report that the resultant reaction milieu catalyses the interconversion of metabolites that in modern organisms constitute glycolysis and the pentose phosphate pathway. The 29 observed reactions include the formation and/or interconversion of glucose, pyruvate, the nucleic acid precursor ribose-5-phosphate and the amino acid precursor erythrose-4-phosphate, antedating reactions sequences similar to that used by the metabolic pathways. Moreover, the Archean ocean mimetic increased the stability of the phosphorylated intermediates and accelerated the rate of intermediate reactions and pyruvate production. The catalytic capacity of the reconstructed ocean milieu was attributable to its metal content. The reactions were particularly sensitive to ferrous iron Fe(II), which is understood to have had high concentrations in the Archean oceans. These observations reveal that reaction sequences that constitute central carbon metabolism could have been constrained by the iron-rich oceanic environment of the early Archean. The origin of metabolism could thus date back to the prebiotic world.
Figures
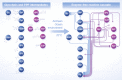
Spontaneous reactivity of glycolytic and pentose phosphate pathway sugar phosphate intermediates as observed in water. One hundred micromolar of the intermediates (the upper panels illustrate the pentose phosphate pathway, the lower panels the combined pentose phosphate pathway and glycolysis) was monitored for 5 h at 70°C. Time‐dependent accumulation of metabolites was considered significant when observed in at least three independent experiments. Arrows connect the substrate with the detected metabolites formed. Every
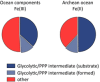
SRM measurement targeted the full spectra of glycolytic and thePPP metabolites. 2‐phosphoglycerate (2PG ) and 3‐phosphoglycerate (3PG ) as well as xylulose 5‐phosphate and ribulose 5‐phosphate could not be separated due to similar retention time, mass and fragmentation spectra and were quantified in pools (grey circles). Metabolites not quantifiable with the approach were 1,3‐bisphosphoglycerate and 6‐phosphoglucono‐δ‐lactone and are omitted from the graph. In total, of the 182 monitored possible reactions, 17 were detected to occur in water. Individual reactions are detailed in Supplementary Table S1; rates are given in Fig 5A.Plausible Archean ocean constituents catalyse metabolism‐like reactions. The same metabolites and reactions were monitored as in (A), with the difference that the reactions were conducted in the presence of Archean ocean plausible concentrations of iron, cobalt, nickel, molybdenum and phosphate. In this milieu, 28 interconversion reactions among glycolytic and pentose phosphate pathway intermediates were observed.
The influence of ferrous iron [Fe(
II )] under anoxic conditions. The same metabolites and reactions were monitored as in (B), with the difference that iron was maintained ferrous Fe(II ), replicating the reducing conditions of the early oceans. In this reaction milieu, 29 metabolite formation reactions were detected. Differences to (B) concern additional interconversion of pentose phosphate metabolites, and fewer interconversions of 3‐carbon metabolites. Individual reactions are detailed in Supplementary Table S1 and Fig 5A.Network topology of modern glycolysis (canonical Embden‐Meyerhof pathway) and the pentose phosphate pathway.

The Archean ocean ionic composition catalyses sugar phosphate interconversions. 6‐phosphogluconate (6
PG ) was incubated at 70°C in water, or in the presence of Archean ocean plausible concentrations ofF e,C o,N i,M o and phosphate. The chromatograms illustrate an exemplaryLC ‐SRM run targeting the glycolytic and pentose phosphate pathway intermediates recorded after 2 h. 6PG was stable in water (upper panel), but was interconverted into other pentose phosphate pathway intermediates and pyruvate as catalysed by the Archean ocean components (lower panel).Iron is the predominant catalyst for pentose phosphate pathway interconversions. 6‐phosphogluconate (6
PG ) and fructose 6‐phosphate (F 6P ) were incubated at 70°C in the presence of the indicated Archean ocean constituents, and the formation of reaction products was monitored byLC ‐SRM over 2 h. The reaction rates were calculated as detailed in the Materials and Methods section; metabolite concentrations are provided in Supplementary Table S5. Ferrous iron facilitated the interconversion of the metabolites into eight metabolic intermediates, whereasC o,N i,M o and phosphate together contributed to a subset of the reactions. Non‐detectable reactions are represented by hatched areas.

The stability of glyceraldehyde 3‐phosphate (
G 3P ) in Archean ocean simulations. G3P was diluted in water, or the Archean ocean mimetic containingF e(III ),C o,N i,M o and phosphate, or the analogous anoxic solution containingF e(II ). The solutions exposed to 70°C and monitored byLC ‐SRM for 5 h. G3P was degraded in water within minutes, was stabilized by the oxygenated, metal‐rich ocean mimetic and remained detectable for more than 5 h in the ferrous iron‐rich ocean simulation.The ferrous iron‐rich Archean ocean ionic composition favours stability of sugar phosphate intermediates. Glycolytic and pentose phosphate pathway intermediates were exposed to 70°C as in (A) and their concentration monitored over 5 h. Illustrated is the fold increase in stability in the Fe(
II )‐rich Archean ocean mimetic over the corresponding stability in the Fe(III )‐rich isoionic solution. All sugar phosphate intermediates that constitute thePPP and glycolysis gained stability. The data obtained in water (Supplementary Tables S2 and S5) are not directly comparable due to the absence of the majority of the pentose phosphate interconversion reactions (Fig 1A and D). Quantitative stability data are given in Supplementary Table S2 and metabolite concentrations in Supplementary Table S5. Error bars indicate ±SD calculated with error propagation.
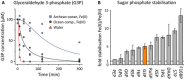
Reaction rates in water (upper panel), and in the presence of ocean components with ferric iron (middle panel), or ferrous iron (Archean ocean, lower panel). The reaction rates in μM/h were determined by monitoring the formation of metabolites over a 5 h time course, n = 3, y‐axis log scaling. Source data are available in Supplementary Table S5.
The reaction rates are expressed relative to the condition with the maximum reaction rate (= 1), to allow direct comparison of the rates in water, and in the Archean mimetics. Non‐detectable reactions are represented by hatched areas. Source data are available in Supplementary Tables S1 and S5.
Reactivity within an Archean ocean mimetic accelerates the enzyme‐free formation of pyruvate. Pyruvate formation rate in a mixture of pentose phosphate pathway and glycolytic intermediates. Glucose 6‐phosphate (G6P), fructose 6‐phosphate (F6P), fructose 1,6‐bisphosphate (F16
BP ), dihydroxyacetone phosphate (DHAP ), glyceraldehyde 3‐phosphate (G3P), 3‐phosphoglycerate (3PG ), phosphoenolpyruvate (PEP ), 6‐phosphogluconate (6PG ), ribulose 5‐phosphate (Ru5P), ribose 5‐phosphate (R5P), xylulose 5‐phosphate (X5P) and sedoheptulose 7‐phosphate (S7P) were each combined at 7.5 μM in: (i) water, (ii) the Archean ocean components with Fe(III ) and (iii) the Archean ocean components in the presence of Fe(II ). The mixture of the metabolic intermediates was exposed for 5 h at 40–90°C in 10°C steps, and pyruvate formation monitored byLC ‐SRM . n = 3, error areas illustrate ±SEM . Pyruvate formation was not detected below 50°C in water and increased in a temperature‐dependent manner, indicative of non‐enzymatic reactions. At all temperatures, pyruvate formation was fastest in the Archean ocean reconstruction; at the model temperature of 70°C (highlighted), accelerated by 200%.
Comment in
-
Prebiotic metabolic networks?Mol Syst Biol. 2014 Apr 25;10(4):729. doi: 10.1002/msb.20145351. Mol Syst Biol. 2014. PMID: 24771086 Free PMC article.
References
-
- Anet FA (2004) The place of metabolism in the origin of life. Curr Opin Chem Biol 8: 654–659 - PubMed
-
- Anastasi C, Buchet FF, Crowe MA, Helliwell M, Raftery J, Sutherland JD (2008) The search for a potentially prebiotic synthesis of nucleotides via arabinose‐3‐phosphate and its cyanamide derivative. Chemistry 14: 2375–2388 - PubMed
-
- Askwith C, Kaplan J (1998) Iron and copper transport in yeast and its relevance to human disease. Trends Biochem Sci 23: 135–138 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

