Regulation of mitochondrial morphology by lipids
- PMID: 24771456
- PMCID: PMC4146713
- DOI: 10.1002/biof.1169
Regulation of mitochondrial morphology by lipids
Abstract
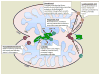
Although great progress has been made in identifying key protein factors that regulate mitochondrial morphology through mediating fission and fusion, signaling lipids are increasingly being recognized as important in the process as well. We review here roles that have been proposed for the signaling and bulk lipids cardiolipin, phosphatidic acid, lysophosphatidic acid, diacylglycerol, and phosphatidylethanolamine and the enzymes that generate or catabolize them in the regulation of mitochondrial morphology in yeast and mammals. Mutations in some of these enzymes are causal in a number of disease settings, highlighting the significance of controlling the lipid environment in this setting.
Keywords: cardiolipin; fission; fusion; mitochondria; phosphatidic acid.
© 2014 International Union of Biochemistry and Molecular Biology.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

