Fosb gene products contribute to excitotoxic microglial activation by regulating the expression of complement C5a receptors in microglia
- PMID: 24771617
- PMCID: PMC4090226
- DOI: 10.1002/glia.22680
Fosb gene products contribute to excitotoxic microglial activation by regulating the expression of complement C5a receptors in microglia
Abstract
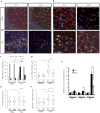
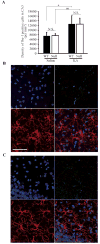
The Fosb gene encodes subunits of the activator protein-1 transcription factor complex. Two mature mRNAs, Fosb and ΔFosb, encoding full-length FOSB and ΔFOSB proteins respectively, are formed by alternative splicing of Fosb mRNA. Fosb products are expressed in several brain regions. Moreover, Fosb-null mice exhibit depressive-like behaviors and adult-onset spontaneous epilepsy, demonstrating important roles in neurological and psychiatric disorders. Study of Fosb products has focused almost exclusively on neurons; their function in glial cells remains to be explored. In this study, we found that microglia express equivalent levels of Fosb and ΔFosb mRNAs to hippocampal neurons and, using microarray analysis, we identified six microglial genes whose expression is dependent on Fosb products. Of these genes, we focused on C5ar1 and C5ar2, which encode receptors for complement C5a. In isolated Fosb-null microglia, chemotactic responsiveness toward the truncated form of C5a was significantly lower than that in wild-type cells. Fosb-null mice were significantly resistant to kainate-induced seizures compared with wild-type mice. C5ar1 mRNA levels and C5aR1 immunoreactivity were increased in wild-type hippocampus 24 hours after kainate administration; however, such induction was significantly reduced in Fosb-null hippocampus. Furthermore, microglial activation after kainate administration was significantly diminished in Fosb-null hippocampus, as shown by significant reductions in CD68 immunoreactivity, morphological change and reduced levels of Il6 and Tnf mRNAs, although no change in the number of Iba-1-positive cells was observed. These findings demonstrate that, under excitotoxicity, Fosb products contribute to a neuroinflammatory response in the hippocampus through regulation of microglial C5ar1 and C5ar2 expression.
Keywords: AP-1 transcription factors; kainic acid; neuroinflammation.
© 2014 Wiley Periodicals, Inc.
Figures

Similar articles
-
fosB-null mice display impaired adult hippocampal neurogenesis and spontaneous epilepsy with depressive behavior.Neuropsychopharmacology. 2013 Apr;38(5):895-906. doi: 10.1038/npp.2012.260. Epub 2012 Dec 18. Neuropsychopharmacology. 2013. PMID: 23303048 Free PMC article.
-
A novel anticonvulsant mechanism via inhibition of complement receptor C5ar1 in murine epilepsy models.Neurobiol Dis. 2015 Apr;76:87-97. doi: 10.1016/j.nbd.2015.02.004. Epub 2015 Feb 11. Neurobiol Dis. 2015. PMID: 25681535
-
Prevention of C5aR1 signaling delays microglial inflammatory polarization, favors clearance pathways and suppresses cognitive loss.Mol Neurodegener. 2017 Sep 18;12(1):66. doi: 10.1186/s13024-017-0210-z. Mol Neurodegener. 2017. PMID: 28923083 Free PMC article.
-
[CEREBRAL MICROGLIA AND MICROGLIAL MARKERS].Morfologiia. 2015;147(3):37-44. Morfologiia. 2015. PMID: 26390545 Review. Russian.
-
Beyond Activation: Characterizing Microglial Functional Phenotypes.Cells. 2021 Aug 28;10(9):2236. doi: 10.3390/cells10092236. Cells. 2021. PMID: 34571885 Free PMC article. Review.
Cited by
-
Epigenetic Regulation of Hippocampal Fosb Expression Controls Behavioral Responses to Cocaine.J Neurosci. 2019 Oct 16;39(42):8305-8314. doi: 10.1523/JNEUROSCI.0800-19.2019. Epub 2019 Sep 2. J Neurosci. 2019. PMID: 31477569 Free PMC article.
-
Single-Cell Analysis of Regional Differences in Adult V-SVZ Neural Stem Cell Lineages.Cell Rep. 2019 Jan 8;26(2):394-406.e5. doi: 10.1016/j.celrep.2018.12.044. Cell Rep. 2019. PMID: 30625322 Free PMC article.
-
Single-Cell Transcriptomic Analyses of Brain Parenchyma in Patients With New-Onset Refractory Status Epilepticus (NORSE).Neurol Neuroimmunol Neuroinflamm. 2024 Jul;11(4):e200259. doi: 10.1212/NXI.0000000000200259. Epub 2024 May 29. Neurol Neuroimmunol Neuroinflamm. 2024. PMID: 38810181 Free PMC article.
-
Modulation of pain sensitivity by Ascl1- and Lhx6-dependent GABAergic neuronal function in streptozotocin diabetic mice.Mol Ther. 2025 Feb 5;33(2):786-804. doi: 10.1016/j.ymthe.2024.12.039. Epub 2024 Dec 30. Mol Ther. 2025. PMID: 39741412 Free PMC article.
-
Single-cell characterization of human GBM reveals regional differences in tumor-infiltrating leukocyte activation.Elife. 2023 Dec 21;12:RP92678. doi: 10.7554/eLife.92678. Elife. 2023. PMID: 38127790 Free PMC article.
References
-
- Anisman H, Hayley S. Inflammatory factors contribute to depression and its comorbid conditions. Sci Signal. 2012;5:pe45. - PubMed
-
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, Yan Z, Sagawa ZK, Ouimet CC, Nairn AC, Nestler EJ, Greengard P. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature. 2001;410:376–380. - PubMed
-
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

