Mitochondrial mitophagy in mesenteric artery remodeling in hyperhomocysteinemia
- PMID: 24771691
- PMCID: PMC4001876
- DOI: 10.14814/phy2.283
Mitochondrial mitophagy in mesenteric artery remodeling in hyperhomocysteinemia
Abstract
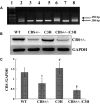
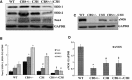
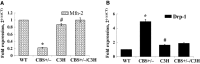
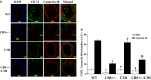
Abstract Although high levels of homocysteine also termed as hyperhomocysteinemia (HHcy) has been associated with inflammatory bowel disease and mesenteric artery occlusion, the mitochondrial mechanisms behind endothelial dysfunction that lead to mesenteric artery remodeling are largely unknown. We hypothesize that in HHcy there is increased mitochondrial fission due to altered Mfn-2/Drp-1 ratio, which leads to endothelial dysfunction and collagen deposition in the mesenteric artery inducing vascular remodeling. To test this hypothesis, we used four groups of mice: (i) WT (C57BL/6J); (ii) mice with HHcy (CBS+/-); (iii) oxidative stress resistant mice (C3H) and (iv) mice with HHcy and oxidative stress resistance (CBS+/-/C3H). For mitochondrial dynamics, we studied the expression of Mfn-2 which is a mitochondrial fusion protein and Drp-1 which is a mitochondrial fission protein by western blots, real-time PCR and immunohistochemistry. We also examined oxidative stress markers, endothelial cell, and gap junction proteins that play an important role in endothelial dysfunction. Our data showed increase in oxidative stress, mitochondrial fission (Drp-1), and collagen deposition in CBS+/- compared to WT and C3H mice. We also observed significant down regulation of Mfn-2 (mitochondrial fusion marker), CD31, eNOS and connexin 40 (gap junction protein) in CBS+/- mice as compared to WT and C3H mice. In conclusion, our data suggested that HHcy increased mitochondrial fission (i.e., decreased Mfn-2/Drp-1 ratio, causing mitophagy) that leads to endothelial cell damage and collagen deposition in the mesenteric artery. This is a novel report on the role of mitochondrial dynamics alteration defining mesenteric artery remodeling.
Keywords: Endothelial dysfunction; hyperhomocysteinemia; mitochondrial dynamics; oxidative stress.
Figures



References
-
- Chambers J. C., McGregor A., Jean‐Marie J., Obeid O. A., Kooner J. S. 1999. Demonstration of rapid onset vascular endothelial dysfunction after hyperhomocysteinemia: an effect reversible with vitamin C therapy. Circulation; 99:1156-1160 - PubMed
-
- Chen C., Conklin B. S., Ren Z., Zhong D. S. 2002. Homocysteine decreases endothelium‐dependent vasorelaxation in porcine arteries. J. Surg. Res.; 102:22-30 - PubMed
-
- Davidson S. M., Duchen M. R. 2007. Endothelial mitochondria: contributing to vascular function and disease. Circ. Res.; 100:1128-1141 - PubMed
-
- De Wit C., Roos F., Bolz S. S., Kirchhoff S., Kruger O., Willecke K. 2000. Impaired conduction of vasodilation along arterioles in connexin40‐deficient mice. Circ. Res.; 86:649-655 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

