Simultaneous imaging of neural activity in three dimensions
- PMID: 24772066
- PMCID: PMC3982072
- DOI: 10.3389/fncir.2014.00029
Simultaneous imaging of neural activity in three dimensions
Abstract
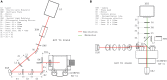
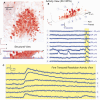
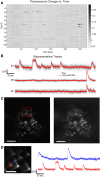
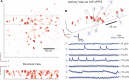
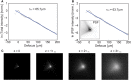
We introduce a scanless optical method to image neuronal activity in three dimensions simultaneously. Using a spatial light modulator and a custom-designed phase mask, we illuminate and collect light simultaneously from different focal planes and perform calcium imaging of neuronal activity in vitro and in vivo. This method, combining structured illumination with volume projection imaging, could be used as a technological platform for brain activity mapping.
Keywords: brain activity map; calcium imaging; spatial-light-modulator; three-dimensional imaging; volume imaging.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

