The contribution of electrical synapses to field potential oscillations in the hippocampal formation
- PMID: 24772068
- PMCID: PMC3982077
- DOI: 10.3389/fncir.2014.00032
The contribution of electrical synapses to field potential oscillations in the hippocampal formation
Abstract
Electrical synapses are a type of cellular membrane junction referred to as gap junctions (GJs). They provide a direct way to exchange ions between coupled cells and have been proposed as a structural basis for fast transmission of electrical potentials between neurons in the brain. For this reason GJs have been regarded as an important component within the neuronal networks that underlie synchronous neuronal activity and field potential oscillations. Initially, GJs appeared to play a particularly key role in the generation of high frequency oscillatory patterns in field potentials. In order to assess the scale of neuronal GJs contribution to field potential oscillations in the hippocampal formation, in vivo and in vitro studies are reviewed here. These investigations have shown that blocking the main neuronal GJs, those containing connexin 36 (Cx36-GJs), or knocking out the Cx36 gene affect field potential oscillatory patterns related to awake active behavior (gamma and theta rhythm) but have no effect on high frequency oscillations occurring during silent wake and sleep. Precisely how Cx36-GJs influence population activity of neurons is more complex than previously thought. Analysis of studies on the properties of transmission through GJ channels as well as Cx36-GJs functioning in pairs of coupled neurons provides some explanations of the specific influence of Cx36-GJs on field potential oscillations. It is proposed here that GJ transmission is strongly modulated by the level of neuronal network activity and changing behavioral states. Therefore, contribution of GJs to field potential oscillatory patterns depends on the behavioral state. I propose here a model, based on large body of experimental data gathered in this field by several authors, in which Cx36-GJ transmission especially contributes to oscillations related to active behavior, where it plays a role in filtering and enhancing coherent signals in the network under high-noise conditions. In contrast, oscillations related to silent wake or sleep, especially high frequency oscillations, do not require transmission by neuronal GJs. The reliability of neuronal discharges during those oscillations could be assured by conditions of higher signal-to-noise ratio and some synaptic changes taking place during active behavior.
Keywords: electrical synapse; fast spiking cells; field potential oscillations; gap junctions; interneurons; neuronal synchronization; parvalbumin interneurons.
Figures
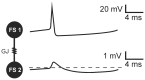
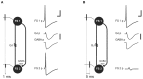

Similar articles
-
The electrical coupling and the hippocampal formation theta rhythm in rats.Brain Res Bull. 2014 Aug;107:1-17. doi: 10.1016/j.brainresbull.2014.04.007. Epub 2014 Apr 18. Brain Res Bull. 2014. PMID: 24747291 Review.
-
Carbenoxolone blockade of neuronal network activity in culture is not mediated by an action on gap junctions.J Physiol. 2003 Dec 15;553(Pt 3):729-45. doi: 10.1113/jphysiol.2003.053439. Epub 2003 Sep 26. J Physiol. 2003. PMID: 14514879 Free PMC article.
-
Neuronal Glutamatergic Network Electrically Wired with Silent But Activatable Gap Junctions.J Neurosci. 2020 Jun 10;40(24):4661-4672. doi: 10.1523/JNEUROSCI.2590-19.2020. Epub 2020 May 11. J Neurosci. 2020. PMID: 32393538 Free PMC article.
-
Interneuronal gap junctions increase synchrony and robustness of hippocampal ripple oscillations.Eur J Neurosci. 2018 Dec;48(12):3446-3465. doi: 10.1111/ejn.14267. Eur J Neurosci. 2018. PMID: 30414336
-
The Roles of Calmodulin and CaMKII in Cx36 Plasticity.Int J Mol Sci. 2021 Apr 25;22(9):4473. doi: 10.3390/ijms22094473. Int J Mol Sci. 2021. PMID: 33922931 Free PMC article. Review.
Cited by
-
Dependence of Generation of Hippocampal CA1 Slow Oscillations on Electrical Synapses.Neurosci Bull. 2020 Jan;36(1):39-48. doi: 10.1007/s12264-019-00419-z. Epub 2019 Aug 29. Neurosci Bull. 2020. PMID: 31468346 Free PMC article.
-
Gap junctions and expression of Cx36, Cx43 and Cx45 in the posterodorsal medial amygdala of adult rats.Histol Histopathol. 2020 Apr;35(4):395-403. doi: 10.14670/HH-18-160. Epub 2019 Sep 9. Histol Histopathol. 2020. PMID: 31495909
-
The Theta Rhythm of the Hippocampus: From Neuronal and Circuit Mechanisms to Behavior.Front Cell Neurosci. 2021 Mar 4;15:649262. doi: 10.3389/fncel.2021.649262. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33746716 Free PMC article.
-
Association of connexin36 with adherens junctions at mixed synapses and distinguishing electrophysiological features of those at mossy fiber terminals in rat ventral hippocampus.Int J Physiol Pathophysiol Pharmacol. 2024 Jun 15;16(3):28-54. doi: 10.62347/RTMH4490. eCollection 2024. Int J Physiol Pathophysiol Pharmacol. 2024. PMID: 39021415 Free PMC article.
-
Facilitation of Hippocampal Kindling and Exacerbation of Kindled Seizures by Intra-CA1 Injection of Quinine: A Possible Role of Cx36 Gap Junctions.Iran Biomed J. 2016 Nov;20(5):266-72. doi: 10.22045/ibj.2016.03. Epub 2016 Apr 25. Iran Biomed J. 2016. PMID: 27108691 Free PMC article.
References
-
- Al-Ubaidi M. R., White T. W., Ripps H., Poras I., Avner P., Gomès D., et al. (2000). Functional properties, developmental regulation, and chromosomal localization of murine connexin36, a gap-junctional protein expressed preferentially in retina and brain. J. Neurosci. Res. 59 813–826 10.1002/(SICI)1097-4547(20000315)59:6<813::AID-JNR14>3.0.CO;2-# - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

