Modeling CaMKII-mediated regulation of L-type Ca(2+) channels and ryanodine receptors in the heart
- PMID: 24772082
- PMCID: PMC3982069
- DOI: 10.3389/fphar.2014.00060
Modeling CaMKII-mediated regulation of L-type Ca(2+) channels and ryanodine receptors in the heart
Abstract
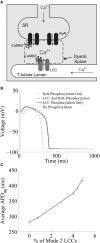
Excitation-contraction coupling (ECC) in the cardiac myocyte is mediated by a number of highly integrated mechanisms of intracellular Ca(2+) transport. Voltage- and Ca(2+)-dependent L-type Ca(2+) channels (LCCs) allow for Ca(2+) entry into the myocyte, which then binds to nearby ryanodine receptors (RyRs) and triggers Ca(2+) release from the sarcoplasmic reticulum in a process known as Ca(2+)-induced Ca(2+) release. The highly coordinated Ca(2+)-mediated interaction between LCCs and RyRs is further regulated by the cardiac isoform of the Ca(2+)/calmodulin-dependent protein kinase (CaMKII). Because CaMKII targets and modulates the function of many ECC proteins, elucidation of its role in ECC and integrative cellular function is challenging and much insight has been gained through the use of detailed computational models. Multiscale models that can both reconstruct the detailed nature of local signaling events within the cardiac dyad and predict their functional consequences at the level of the whole cell have played an important role in advancing our understanding of CaMKII function in ECC. Here, we review experimentally based models of CaMKII function with a focus on LCC and RyR regulation, and the mechanistic insights that have been gained through their application.
Keywords: CaMKII; cardiac myocyte; cell signaling; computational modeling; excitation-contraction coupling.
Figures

References
-
- Bers D. M. (2001). Excitation-Contraction Coupling and Cardiac Contractile Force. Boston, MA: Kluwer; 10.1007/978-94-010-0658-3 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

