Regulation of soluble guanylate cyclase by matricellular thrombospondins: implications for blood flow
- PMID: 24772092
- PMCID: PMC3983488
- DOI: 10.3389/fphys.2014.00134
Regulation of soluble guanylate cyclase by matricellular thrombospondins: implications for blood flow
Abstract
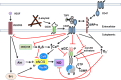
Nitric oxide (NO) maintains cardiovascular health by activating soluble guanylate cyclase (sGC) to increase cellular cGMP levels. Cardiovascular disease is characterized by decreased NO-sGC-cGMP signaling. Pharmacological activators and stimulators of sGC are being actively pursued as therapies for acute heart failure and pulmonary hypertension. Here we review molecular mechanisms that modulate sGC activity while emphasizing a novel biochemical pathway in which binding of the matricellular protein thrombospondin-1 (TSP1) to the cell surface receptor CD47 causes inhibition of sGC. We discuss the therapeutic implications of this pathway for blood flow, tissue perfusion, and cell survival under physiologic and disease conditions.
Keywords: CD47; ROS; cardiovascular disease; cyclic guanosine monophosphate; nitric oxide; soluble guanylate cyclase; thrombospondin-1.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

