Quantitative tumor segmentation for evaluation of extent of glioblastoma resection to facilitate multisite clinical trials
- PMID: 24772206
- PMCID: PMC3998691
- DOI: 10.1593/tlo.13835
Quantitative tumor segmentation for evaluation of extent of glioblastoma resection to facilitate multisite clinical trials
Abstract
Standard-of-care therapy for glioblastomas, the most common and aggressive primary adult brain neoplasm, is maximal safe resection, followed by radiation and chemotherapy. Because maximizing resection may be beneficial for these patients, improving tumor extent of resection (EOR) with methods such as intraoperative 5-aminolevulinic acid fluorescence-guided surgery (FGS) is currently under evaluation. However, it is difficult to reproducibly judge EOR in these studies due to the lack of reliable tumor segmentation methods, especially for postoperative magnetic resonance imaging (MRI) scans. Therefore, a reliable, easily distributable segmentation method is needed to permit valid comparison, especially across multiple sites. We report a segmentation method that combines versatile region-of-interest blob generation with automated clustering methods. We applied this to glioblastoma cases undergoing FGS and matched controls to illustrate the method's reliability and accuracy. Agreement and interrater variability between segmentations were assessed using the concordance correlation coefficient, and spatial accuracy was determined using the Dice similarity index and mean Euclidean distance. Fuzzy C-means clustering with three classes was the best performing method, generating volumes with high agreement with manual contouring and high interrater agreement preoperatively and postoperatively. The proposed segmentation method allows tumor volume measurements of contrast-enhanced T 1-weighted images in the unbiased, reproducible fashion necessary for quantifying EOR in multicenter trials.
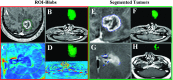
Figures



References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJB, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. - PubMed
-
- Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. - PubMed
-
- Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
