Phase II Trial of Cetuximab and Conformal Radiotherapy Only in Locally Advanced Pancreatic Cancer with Concurrent Tissue Sampling Feasibility Study
- PMID: 24772208
- PMCID: PMC3998695
- DOI: 10.1593/tlo.13724
Phase II Trial of Cetuximab and Conformal Radiotherapy Only in Locally Advanced Pancreatic Cancer with Concurrent Tissue Sampling Feasibility Study
Abstract
Background: Preclinical data have indicated the anti-epidermal growth factor receptor (EGFR) agent cetuximab (Erbitux) as a radiosensitizer in pancreatic cancer, but this has not been specifically addressed in a clinical study. We report the results of an original study initiated in 2007, where cetuximab was tested with radiotherapy (RT) alone in locally advanced pancreatic cancer in a phase II trial (PACER).
Methods: Patients (n = 21) received cetuximab loading dose (400 mg/m(2)) and weekly dose (250 mg/m(2)) during RT (50.4 Gy in 28 fractions). Toxicity and disease response end point data were prospectively assessed. A feasibility study of on-trial patient blood and skin sampling was incorporated.
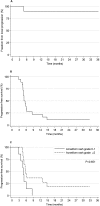
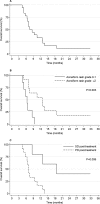
Results: Treatment was well tolerated, and toxicity was low; most patients (71%) experienced acute toxicities of grade 2 or less. Six months posttreatment, stable local disease was achieved in 90% of evaluable patients, but only 33% were free from metastatic progression. Median overall survival was 7.5 months, and actuarial survival was 33% at 1 year and 11% at 3 years, reflecting swift metastatic progression in some patients but good long-term control of localized disease in others. High-grade acneiform rash (P = .0027), posttreatment stable disease (P = .0059), and pretreatment cancer antigen 19.9 (CA19.9) level (P = .0042) associated with extended survival. Patient skin and blood samples yielded sufficient RNA and good quality protein, respectively.
Conclusions: The results indicate that cetuximab inhibits EGFR-mediated radioresistance to achieve excellent local control with minimal toxicity but does not sufficiently control metastatic progression in all patients. Translational studies of patient tissue samples may yield molecular information that may enable individual treatment response prediction.
Figures

References
-
- Crane CH, Varadhachary G, Settle SH, Fleming JB, Evans DB, Wolff RA. The integration of chemoradiation in the care of patient with localized pancreatic cancer. Cancer Radiother. 2009;13:123–143. - PubMed
-
- NICE, author. Guidance on the Use of Gemcitabine for the Treatment of Pancreatic Cancer. National Institute of Clinical Excellence (NICE); 2001.
-
- Ben-Josef E, Schipper M, Francis IR, Hadley S, Ten-Haken R, Lawrence T, Normolle D, Simeone DM, Sonnenday C, Abrams R, et al. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys. 2012;84:1166–1171. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
