Phase II study of concurrent chemoradiotherapy with carboplatin and vinorelbine for locally advanced non-small-cell lung cancer
- PMID: 24772308
- PMCID: PMC3999118
- DOI: 10.3892/mco.2014.252
Phase II study of concurrent chemoradiotherapy with carboplatin and vinorelbine for locally advanced non-small-cell lung cancer
Abstract
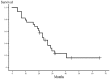
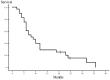
Patients with non-small cell lung cancer (NSCLC) have locally advanced disease with poor prognosis. Although concurrent chemoradiotherapy is the standard treatment, more effective regimens are required. The aim of this study was to assess the safety and efficacy of concurrent chemoradiotherapy with a divided schedule of carboplatin and vinorelbine in patients with locally advanced NSCLC. Patients with unresectable, stage IIIA or IIIB NSCLC were eligible for enrollment if they exhibited a performance status of 0-2 and were ≤75 years of age. Patients were treated with carboplatin at an area under the plasma concentration vs. time curve of 2.5 mg/ml/min and vinorelbine at 20 mg/m2 on days 1 and 8 every 3 weeks. Thoracic radiotherapy at a total dose of 60 Gy was concurrently administered (2 Gy per fraction). Twenty-eight patients (23 men and 5 women; median age, 67 years; range 47-75 years) were enrolled in the present study. The overall response rate was 85.7% [95% confidence interval (CI), 67.3-96.0%] and the disease control rate was 96.4% (95% CI, 81.7-99.9%). The median survival time (MST) was 23 months and the median progression-free survival (PFS) time was 8 months. Grade 3-4 toxicities included neutropenia, thrombocytopenia, anemia and infection in 100, 14, 46 and 36% of patients, respectively. One patient (4%) developed grade 3 radiation esophagitis that resolved completely without residual dilation. Grade 3 radiation pneumonitis occurred in 2 patients (7%); however, the symptoms and radiographic abnormalities subsided with corticosteroid therapy. In conclusion, concurrent chemoradiotherapy with a divided schedule of carboplatin and vinorelbine is well-tolerated and effective in patients with locally advanced NSCLC.
Keywords: carboplatin; chemoradiotherapy; non-small-cell lung cancer; vinorelbine.
Figures
References
-
- Goldstraw P, Crowley J, Chansky K, et al. International Association for the Study of Lung Cancer International Staging Committee and Participating Institutions. The IASCL Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–714. - PubMed
-
- Auperin A, Le Pechoux C, Pignon JP, et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Ann Oncol. 2006;17:473–483. - PubMed
-
- Auperin A, Le Pechoux C, Rolland E, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–2190. - PubMed
-
- Vokes EE, Herndon JE, II, Crawford J. Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non-small-cell lung cancer: cancer and leukemia group B study 9431. J Clin Oncol. 2002;20:4191–4198. - PubMed
-
- Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol. 1999;17:409–422. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources