The general anaesthetic etomidate inhibits the excitability of mouse thalamocortical relay neurons by modulating multiple modes of GABAA receptor-mediated inhibition
- PMID: 24773078
- PMCID: PMC4215602
- DOI: 10.1111/ejn.12601
The general anaesthetic etomidate inhibits the excitability of mouse thalamocortical relay neurons by modulating multiple modes of GABAA receptor-mediated inhibition
Abstract
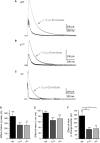
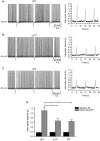
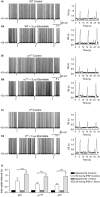
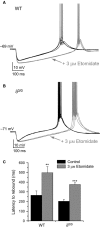
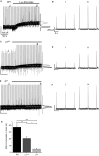
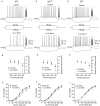
Modulation of thalamocortical (TC) relay neuron function has been implicated in the sedative and hypnotic effects of general anaesthetics. Inhibition of TC neurons is mediated predominantly by a combination of phasic and tonic inhibition, together with a recently described 'spillover' mode of inhibition, generated by the dynamic recruitment of extrasynaptic γ-aminobutyric acid (GABA)A receptors (GABAA Rs). Previous studies demonstrated that the intravenous anaesthetic etomidate enhances tonic and phasic inhibition in TC relay neurons, but it is not known how etomidate may influence spillover inhibition. Moreover, it is unclear how etomidate influences the excitability of TC neurons. Thus, to investigate the relative contribution of synaptic (α1β2γ2) and extrasynaptic (α4β2δ) GABAA Rs to the thalamic effects of etomidate, we performed whole-cell recordings from mouse TC neurons lacking synaptic (α1(0/0) ) or extrasynaptic (δ(0/0) ) GABAA Rs. Etomidate (3 μm) significantly inhibited action-potential discharge in a manner that was dependent on facilitation of both synaptic and extrasynaptic GABAA Rs, although enhanced tonic inhibition was dominant in this respect. Additionally, phasic inhibition evoked by stimulation of the nucleus reticularis exhibited a spillover component mediated by δ-GABAA Rs, which was significantly prolonged in the presence of etomidate. Thus, etomidate greatly enhanced the transient suppression of TC spike trains by evoked inhibitory postsynaptic potentials. Collectively, these results suggest that the deactivation of thalamus observed during etomidate-induced anaesthesia involves potentiation of tonic and phasic inhibition, and implicate amplification of spillover inhibition as a novel mechanism to regulate the gating of sensory information through the thalamus during anaesthetic states.
Keywords: nucleus reticularis; phasic inhibition; spill-over inhibition; thalamus; tonic inhibition.
© 2014 The Authors. European Journal of Neuroscience published by Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Figures

Similar articles
-
Developmentally regulated neurosteroid synthesis enhances GABAergic neurotransmission in mouse thalamocortical neurones.J Physiol. 2015 Jan 1;593(1):267-84. doi: 10.1113/jphysiol.2014.280263. Epub 2014 Dec 3. J Physiol. 2015. PMID: 25556800 Free PMC article.
-
Extrasynaptic GABA(A) receptors couple presynaptic activity to postsynaptic inhibition in the somatosensory thalamus.J Neurosci. 2013 Sep 11;33(37):14850-68. doi: 10.1523/JNEUROSCI.1174-13.2013. J Neurosci. 2013. PMID: 24027285 Free PMC article.
-
Modeling the GABAergic action of etomidate on the thalamocortical system.Anesth Analg. 2009 Jan;108(1):160-7. doi: 10.1213/ane.0b013e31818d40aa. Anesth Analg. 2009. PMID: 19095844 Free PMC article.
-
GABAA receptors in the thalamus: alpha4 subunit expression and alcohol sensitivity.Alcohol. 2007 May;41(3):177-85. doi: 10.1016/j.alcohol.2007.03.010. Epub 2007 May 23. Alcohol. 2007. PMID: 17521848 Review.
-
Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses.Pharmacol Ther. 2004 Jun;102(3):195-221. doi: 10.1016/j.pharmthera.2004.04.003. Pharmacol Ther. 2004. PMID: 15246246 Review.
Cited by
-
Developmentally regulated neurosteroid synthesis enhances GABAergic neurotransmission in mouse thalamocortical neurones.J Physiol. 2015 Jan 1;593(1):267-84. doi: 10.1113/jphysiol.2014.280263. Epub 2014 Dec 3. J Physiol. 2015. PMID: 25556800 Free PMC article.
-
Comparison of αβδ and αβγ GABAA receptors: Allosteric modulation and identification of subunit arrangement by site-selective general anesthetics.Pharmacol Res. 2018 Jul;133:289-300. doi: 10.1016/j.phrs.2017.12.031. Epub 2017 Dec 30. Pharmacol Res. 2018. PMID: 29294355 Free PMC article. Review.
-
Realising the therapeutic potential of neuroactive steroid modulators of the GABAA receptor.Neurobiol Stress. 2019 Dec 23;12:100207. doi: 10.1016/j.ynstr.2019.100207. eCollection 2020 May. Neurobiol Stress. 2019. PMID: 32435660 Free PMC article.
-
Auditory thalamic circuits and GABAA receptor function: Putative mechanisms in tinnitus pathology.Hear Res. 2017 Jun;349:197-207. doi: 10.1016/j.heares.2016.08.009. Epub 2016 Aug 21. Hear Res. 2017. PMID: 27553899 Free PMC article. Review.
-
Opposing actions of CRF-R1 and CB1 receptor on facial stimulation-induced MLI-PC plasticity in mouse cerebellar cortex.BMC Neurosci. 2022 Jun 26;23(1):39. doi: 10.1186/s12868-022-00726-8. BMC Neurosci. 2022. PMID: 35754033 Free PMC article.
References
-
- Alkire MT, Haier RJ. Fallon JH. Toward a unified theory of narcosis: brain imaging evidence for a thalamocortical switch as the neurophysiologic basis of anesthetic-induced unconsciousness. Conscious. Cogn. 2000;9:370–386. - PubMed
-
- Andrada J, Livingston P, Lee BJ. Antognini J. Propofol and etomidate depress cortical, thalamic, and reticular formation neurons during anesthetic-induced unconsciousness. Anesth. Analg. 2012;114:661–669. - PubMed
-
- Angel A. The G. L. Brown lecture. Adventures in anaesthesia. Exp. Physiol. 1991;76:1–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

