Clinical findings and prevalence of the mutation associated with primary ciliary dyskinesia in Old English Sheepdogs
- PMID: 24773602
- PMCID: PMC4895470
- DOI: 10.1111/jvim.12336
Clinical findings and prevalence of the mutation associated with primary ciliary dyskinesia in Old English Sheepdogs
Abstract
Background: Primary ciliary dyskinesia (PCD) is generally a recessively inherited disorder characterized by dysfunction of motile cilia. A mutation in a new causative gene (CCDC39) has been identified in the Old English Sheepdog (OES).
Objectives: To describe the clinical findings and the molecular changes of affected dogs and estimate the worldwide prevalence of the mutation in a large cohort of OES.
Animals: 578 OES, including 28 affected and 550 clinically healthy dogs.
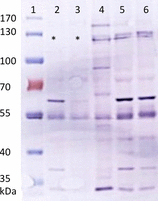
Methods: This retrospective study reviewed the data of OES diagnosed with PCD and OES tested for the mutation. Clinical data including results of physical examination and further investigations were obtained on 11/28 dogs. CCDC39 expression was assessed by qRT-PCR and Western blot analysis in affected dogs and healthy dogs. DNA was extracted on 561/578 dogs and a genetic test by Taqman technology was developed to genotype the CCDC39 mutation in these dogs.
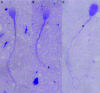
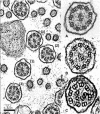
Results: Clinical findings were recurrent nasal discharge and cough, pyrexia, leucocytosis, and bronchopneumonia. Ultrastructural defects were characterized by central microtubular abnormalities and decreased number of inner dynein arms (IDAs). Molecular analysis revealed a reduced expression of CCDC39 RNA and an absence of CCDC39 protein in affected dogs compared to healthy dogs. The mutation was more frequent in nonrandomly selected European OES population with a higher proportion of carriers (19%) compared to non-European dogs (7%).
Conclusion and clinical importance: CCDC39 mutation is dispersed in a worldwide population and is responsible for PCD in this breed. Genetic testing might enable control of this disease.
Keywords: Bronchopneumonia; CCDC39; Ciliary dysfunction; Dog; Genetic.
Copyright © 2014 by the American College of Veterinary Internal Medicine.
Figures


Similar articles
-
Biallelic Variants in CCDC39 Gene Lead to Primary Ciliary Dyskinesia and Kartagener Syndrome.Biomed Res Int. 2022 Jun 26;2022:7130555. doi: 10.1155/2022/7130555. eCollection 2022. Biomed Res Int. 2022. PMID: 35795318 Free PMC article.
-
Histologic and Ultrastructural Findings in Dogs With Chronic Respiratory Disease Suspected of Ciliary Dyskinesia.Vet Pathol. 2017 Sep;54(5):802-812. doi: 10.1177/0300985817705170. Epub 2017 May 11. Vet Pathol. 2017. PMID: 28494707
-
Delineation of CCDC39/CCDC40 mutation spectrum and associated phenotypes in primary ciliary dyskinesia.J Med Genet. 2012 Jun;49(6):410-6. doi: 10.1136/jmedgenet-2012-100867. J Med Genet. 2012. PMID: 22693285
-
[Cilia ultrastructural and gene variation of primary ciliary dyskinesia: report of three cases and literatures review].Zhonghua Er Ke Za Zhi. 2018 Feb 2;56(2):134-137. doi: 10.3760/cma.j.issn.0578-1310.2018.02.012. Zhonghua Er Ke Za Zhi. 2018. PMID: 29429202 Review. Chinese.
-
Value of transmission electron microscopy for primary ciliary dyskinesia diagnosis in the era of molecular medicine: Genetic defects with normal and non-diagnostic ciliary ultrastructure.Ultrastruct Pathol. 2017 Nov-Dec;41(6):373-385. doi: 10.1080/01913123.2017.1362088. Epub 2017 Sep 15. Ultrastruct Pathol. 2017. PMID: 28915070 Free PMC article. Review.
Cited by
-
Recognition and Diagnosis of Underlying Disease Processes in Bacterial Pneumonia.Animals (Basel). 2024 May 29;14(11):1601. doi: 10.3390/ani14111601. Animals (Basel). 2024. PMID: 38891647 Free PMC article. Review.
-
Siewert-Kartagener's syndrome in a dog.J Vet Sci. 2023 Jul;24(4):e57. doi: 10.4142/jvs.23029. J Vet Sci. 2023. PMID: 37532300 Free PMC article.
-
Recurrent bacterial pneumonia in Irish Wolfhounds: Clinical findings and etiological studies.J Vet Intern Med. 2019 Mar;33(2):846-855. doi: 10.1111/jvim.15413. Epub 2019 Jan 21. J Vet Intern Med. 2019. PMID: 30666726 Free PMC article.
-
Host-microbe interactions in the nasal cavity of dogs with chronic idiopathic rhinitis.Front Vet Sci. 2024 Aug 12;11:1385471. doi: 10.3389/fvets.2024.1385471. eCollection 2024. Front Vet Sci. 2024. PMID: 39188898 Free PMC article.
-
NME5 frameshift variant in Alaskan Malamutes with primary ciliary dyskinesia.PLoS Genet. 2019 Sep 3;15(9):e1008378. doi: 10.1371/journal.pgen.1008378. eCollection 2019 Sep. PLoS Genet. 2019. PMID: 31479451 Free PMC article.
References
-
- Zariwala MA, Knowles MR, Omran H. Genetic defects in ciliary structure and function. Annu Rev Physiol 2007;69:423–450. - PubMed
-
- Ibanez‐Tallon I, Heintz N, Omran H. To beat or not to beat: Roles of cilia in development and disease. Hum Mol Genet 2003;12:R27–R35. - PubMed
-
- Clercx C, Peeters D, Beths T, et al. Use of ciliogenesis in the diagnosis of primary ciliary dyskinesia in a dog. J Am Vet Med Assoc 2000;217:1681–1685, 1659. - PubMed
-
- Edwards DF, Patton CS, Kennedy JR. Primary ciliary dyskinesia in the dog. Probl Vet Med 1992;4:291–319. - PubMed
-
- De Scally M, Lobetti RG, Van Wilpe E. Primary ciliary dyskinesia in a Staffordshire Bull Terrier. J S Afr Vet Assoc 2004;75:150–152. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

