Atrial fibrosis in a chronic murine model of obstructive sleep apnea: mechanisms and prevention by mesenchymal stem cells
- PMID: 24775918
- PMCID: PMC4012097
- DOI: 10.1186/1465-9921-15-54
Atrial fibrosis in a chronic murine model of obstructive sleep apnea: mechanisms and prevention by mesenchymal stem cells
Abstract
Background: OSA increases atrial fibrillation (AF) risk and is associated with poor AF treatment outcomes. However, a causal association is not firmly established and the mechanisms involved are poorly understood. The aims of this work were to determine whether chronic obstructive sleep apnea (OSA) induces an atrial pro-arrhythmogenic substrate and to explore whether mesenchymal stem cells (MSC) are able to prevent it in a rat model of OSA.
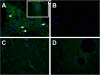
Methods: A custom-made setup was used to mimic recurrent OSA-like airway obstructions in rats. OSA-rats (n = 16) were subjected to 15-second obstructions, 60 apneas/hour, 6 hours/day during 21 consecutive days. Sham rats (n = 14) were placed in the setup but no obstructions were applied. In a second series of rats, MSC were administered to OSA-rats and saline to Sham-rats. Myocardial collagen deposit was evaluated in Picrosirius-red stained samples. mRNA expression of genes involved in collagen turnover, inflammation and oxidative stress were quantified by real time PCR. MMP-2 protein levels were quantified by Western Blot.
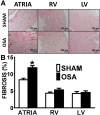
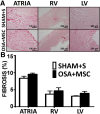
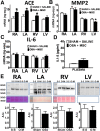
Results: A 43% greater interstitial collagen fraction was observed in the atria, but not in the ventricles, of OSA-rats compared to Sham-rats (Sham 8.32 ± 0.46% vs OSA 11.90 ± 0.59%, P < 0.01). Angiotensin-I Converting Enzyme (ACE) and Interleukin 6 (IL-6) expression were significantly increased in both atria, while Matrix Metalloproteinase-2 (MMP-2) expression was decreased. MSC administration blunted OSA-induced atrial fibrosis (Sham + Saline 8.39 ± 0.56% vs OSA + MSC 9.57 ± 0.31%, P = 0.11), as well as changes in MMP-2 and IL-6 expression. Interleukin 1-β (IL-1β) plasma concentration correlated to atrial but not ventricular fibrosis. Notably, a 2.5-fold increase in IL-1β plasma levels was observed in the OSA group, which was prevented in rats receiving MSC.
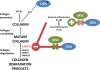
Conclusions: OSA induces selective atrial fibrosis in a chronic murine model, which can be mediated in part by the systemic and local inflammation and by decreased collagen-degradation. MSCs transplantation prevents atrial fibrosis, suggesting that these stem cells could counterbalance inflammation in OSA.
Figures

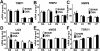


References
-
- Kanagala R, Murali NS, Friedman PA, Ammash NM, Gersh BJ, Ballman KV, Shamsuzzaman AS, Somers VK. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–2594. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

