BRCA1 controls homologous recombination at Tus/Ter-stalled mammalian replication forks
- PMID: 24776801
- PMCID: PMC4118467
- DOI: 10.1038/nature13295
BRCA1 controls homologous recombination at Tus/Ter-stalled mammalian replication forks
Abstract
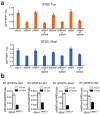
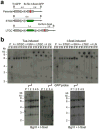
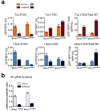
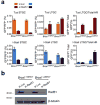
Replication fork stalling can promote genomic instability, predisposing to cancer and other diseases. Stalled replication forks may be processed by sister chromatid recombination (SCR), generating error-free or error-prone homologous recombination (HR) outcomes. In mammalian cells, a long-standing hypothesis proposes that the major hereditary breast/ovarian cancer predisposition gene products, BRCA1 and BRCA2, control HR/SCR at stalled replication forks. Although BRCA1 and BRCA2 affect replication fork processing, direct evidence that BRCA gene products regulate homologous recombination at stalled chromosomal replication forks is lacking, due to a dearth of tools for studying this process. Here we report that the Escherichia coli Tus/Ter complex can be engineered to induce site-specific replication fork stalling and chromosomal HR/SCR in mouse cells. Tus/Ter-induced homologous recombination entails processing of bidirectionally arrested forks. We find that the Brca1 carboxy (C)-terminal tandem BRCT repeat and regions of Brca1 encoded by exon 11-two Brca1 elements implicated in tumour suppression-control Tus/Ter-induced homologous recombination. Inactivation of either Brca1 or Brca2 increases the absolute frequency of 'long-tract' gene conversions at Tus/Ter-stalled forks, an outcome not observed in response to a site-specific endonuclease-mediated chromosomal double-strand break. Therefore, homologous recombination at stalled forks is regulated differently from homologous recombination at double-strand breaks arising independently of a replication fork. We propose that aberrant long-tract homologous recombination at stalled replication forks contributes to genomic instability and breast/ovarian cancer predisposition in BRCA mutant cells.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures








References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

