T cells expressing chimeric antigen receptors can cause anaphylaxis in humans
- PMID: 24777247
- PMCID: PMC3888798
- DOI: 10.1158/2326-6066.CIR-13-0006
T cells expressing chimeric antigen receptors can cause anaphylaxis in humans
Abstract
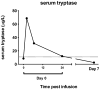
T cells can be redirected to overcome tolerance to cancer by engineering with integrating vectors to express a chimeric antigen receptor (CAR). In preclinical models, we have previously shown that transfection of T cells with mRNA coding for a CAR is an alternative strategy that has antitumor efficacy and the potential to evaluate the on-target off-tumor toxicity of new CAR targets safely due to transient mRNA CAR expression. Here, we report the safety observed in four patients treated with autologous T cells that had been electroporated with mRNA coding for a CAR derived from a murine antibody to human mesothelin. Because of the transient nature of CAR expression on the T cells, subjects in the clinical study were given repeated infusions of the CAR-T cells to assess their safety. One subject developed anaphylaxis and cardiac arrest within minutes of completing the third infusion. Although human anti-mouse immunoglobulin (Ig)G antibodies have been known to develop with CAR-transduced T cells, they have been thought to have no adverse clinical consequences. This is the first description of clinical anaphylaxis resulting from CAR-modified T cells, most likely through IgE antibodies specific to the CAR. These results indicate that the potential immunogenicity of CARs derived from murine antibodies may be a safety issue for mRNA CARs, especially when administered using an intermittent dosing schedule.
Trial registration: ClinicalTrials.gov NCT01355965.
©2013 AACR.
Conflict of interest statement
Some authors have intellectual property for chimeric antigen receptor technology (BLL, YZ, CHJ and MK). The other authors have no conflict.
Figures


References
-
- Davis JL, Theoret MR, Zheng Z, Lamers CH, Rosenberg SA, Morgan RA. Development of human anti-murine T-cell receptor antibodies in both responding and nonresponding patients enrolled in TCR gene therapy trials. Clinical cancer research: an official journal of the American Association for Cancer Research. 2010;16:5852–61. - PMC - PubMed
-
- Chang K, Pai LH, Batra JK, Pastan I, Willingham MC. Characterization of the antigen (CAK1) recognized by monoclonal antibody K1 present on ovarian cancers and normal mesothelium. Cancer research. 1992;52:181–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

