Drug and gene delivery to the back of the eye: from bench to bedside
- PMID: 24777644
- PMCID: PMC4004426
- DOI: 10.1167/iovs.13-13707
Drug and gene delivery to the back of the eye: from bench to bedside
Keywords: drug delivery; gene therapy; nanoparticle; retina; translational medicine.
Figures
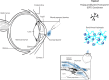

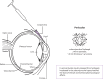


References
-
- Gordois A, Pezzullo L, Cutler H. The Global Economic Cost of Visual Impairment. Sidney: Access Economics Pty Limited; 2010.
-
- Sebag J. Age-related changes in human vitreous structure. Graefes Arch Clin Exp Ophthalmol. 1987; 225: 89–93 - PubMed
-
- Hughes PM, Olejnik O, Chang-Lin JE, Wilson CG. Topical and systemic drug delivery to the posterior segment. Adv Drug Deliv Rev. 2005; 57: 2010–2032 - PubMed
-
- Bill A. Movement of albumin and dextran through the sclera. Arch Ophthalmol. 1965; 74: 248–252 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

