Digital drug safety surveillance: monitoring pharmaceutical products in twitter
- PMID: 24777653
- PMCID: PMC4013443
- DOI: 10.1007/s40264-014-0155-x
Digital drug safety surveillance: monitoring pharmaceutical products in twitter
Erratum in
- Drug Saf. 2014 Jul;37(7):555
Abstract
Background: Traditional adverse event (AE) reporting systems have been slow in adapting to online AE reporting from patients, relying instead on gatekeepers, such as clinicians and drug safety groups, to verify each potential event. In the meantime, increasing numbers of patients have turned to social media to share their experiences with drugs, medical devices, and vaccines.
Objective: The aim of the study was to evaluate the level of concordance between Twitter posts mentioning AE-like reactions and spontaneous reports received by a regulatory agency.
Methods: We collected public English-language Twitter posts mentioning 23 medical products from 1 November 2012 through 31 May 2013. Data were filtered using a semi-automated process to identify posts with resemblance to AEs (Proto-AEs). A dictionary was developed to translate Internet vernacular to a standardized regulatory ontology for analysis (MedDRA(®)). Aggregated frequency of identified product-event pairs was then compared with data from the public FDA Adverse Event Reporting System (FAERS) by System Organ Class (SOC).
Results: Of the 6.9 million Twitter posts collected, 4,401 Proto-AEs were identified out of 60,000 examined. Automated, dictionary-based symptom classification had 86 % recall and 72 % precision [corrected]. Similar overall distribution profiles were observed, with Spearman rank correlation rho of 0.75 (p < 0.0001) between Proto-AEs reported in Twitter and FAERS by SOC.
Conclusion: Patients reporting AEs on Twitter showed a range of sophistication when describing their experience. Despite the public availability of these data, their appropriate role in pharmacovigilance has not been established. Additional work is needed to improve data acquisition and automation.
Figures


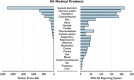
References
-
- Duggirala HJ, Herz ND, Caños DA, Sullivan RA, Schaaf R, Pinnow E, et al. Disproportionality analysis for signal detection of implantable cardioverter-defibrillator-related adverse events in the Food and Drug Administration Medical Device Reporting System. Pharmacoepidemiol Drug Saf. 2012;21:87–93. doi: 10.1002/pds.2261. - DOI - PubMed
-
- Levinson D. Hospital incident reporting systems do not capture most patient harm (OEI-06-09-00091) 01-05-2012 [Internet]. Cited 17 Oct 2013. http://oig.hhs.gov/oei/reports/oei-06-09-00091.pdf.
-
- Center for Drug Evaluation, Research. FDA adverse events reporting system (FAERS)—FDA adverse event reporting system (FAERS): latest quarterly data files. fda.gov. Center for drug evaluation and research.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

