Enhanced activation of matrix metalloproteinase-9 correlates with the degree of papillary thyroid carcinoma infiltration
- PMID: 24778099
- PMCID: PMC4009713
- DOI: 10.3325/cmj.2014.55.128
Enhanced activation of matrix metalloproteinase-9 correlates with the degree of papillary thyroid carcinoma infiltration
Abstract
Aim: To determine whether matrix metalloproteinase-9 (MMP-9) may be a useful adjunctive tool for predicting unfavorable biological behavior of papillary thyroid carcinoma (PTC) by evaluating the expression profile and proteolytic activity of MMP-9 in PTC by different techniques and correlating the findings with clinicopathological prognostic factors.
Methods: Immunohistochemical localization of MMP-9 was analyzed with antibodies specific for either total or active MMP-9. Activation ratios of MMP-9 were calculated by quantifying gel zymography bands. Enzymatic activity of MMP-9 was localized by in situ zymography after inhibiting MMP-2 activity.
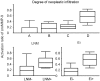
Results: Immunostaining of total and active MMP-9 was observed in tumor tissue and occasionally in non-neoplastic epithelium. Only active MMP-9 was significantly associated with extrathyroid invasion, lymph-node metastasis, and the degree of tumor infiltration (P<0.001, P=0.004, and P<0.001, respectively). Gelatin zymography revealed a correlation between the MMP-9 activation ratio and nodal involvement, extrathyroid invasion, and the degree of tumor infiltration. In situ zymography showed that gelatinases exerted their activity in tumor parenchymal and stromal cells. Moreover, after application of MMP-2 inhibitor, the remaining gelatinase activity, corresponding to MMP-9, was highest in cancers with the most advanced degree of tumor infiltration.
Conclusions: This is the first report suggesting that the evaluation of active MMP-9 by immunohistochemistry and determination of its activation ratio by gelatin zymography may be a useful adjunct to the known clinicopathological factors in predicting tumor behavior. Most important, in situ zimography with an MMP-2 inhibitor for the first time demonstrated a strong impact of MMP-9 activity on the degree of tumor infiltration during PTC progression.
Figures




References
-
- Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, et al. American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. - DOI - PubMed
-
- Björklund M, Koivunen E. Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta. 2005;1755:37–69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous