Characterization of immunological cross-reactivity between enterotoxigenic Escherichia coli heat-stable toxin and human guanylin and uroguanylin
- PMID: 24778111
- PMCID: PMC4097616
- DOI: 10.1128/IAI.01749-14
Characterization of immunological cross-reactivity between enterotoxigenic Escherichia coli heat-stable toxin and human guanylin and uroguanylin
Abstract
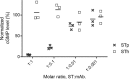
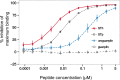
Enterotoxigenic Escherichia coli (ETEC) expressing the heat-stable toxin (ST) (human-type [STh] and porcine-type [STp] variants) is among the five most important enteric pathogens in young children living in low- and middle-income countries. ST mediates diarrheal disease through activation of the guanylate cyclase C (GC-C) receptor and is an attractive vaccine target with the potential to confer protection against a wide range of ETEC strains. However, immunological cross-reactivity to the endogenous GC-C ligands guanylin and uroguanylin is a major concern because of the similarities to ST in amino acid sequence, structure, and function. We have investigated the presence of similar epitopes on STh, STp, guanylin, and uroguanylin by analyzing these peptides in eight distinct competitive enzyme-linked immunosorbent assays (ELISAs). A fraction (27%) of a polyclonal anti-STh antibody and an anti-STh monoclonal antibody (MAb) cross-reacted with uroguanylin, the latter with a 73-fold-lower affinity. In contrast, none of the antibodies raised against STp, one polyclonal antibody and three MAbs, cross-reacted with the endogenous peptides. Antibodies raised against guanylin and uroguanylin showed partial cross-reactivity with the ST peptides. Our results demonstrate, for the first time, that immunological cross-reactions between ST and the endogenous peptides can occur. However, the partial nature and low affinity of the observed cross-reactions suggest that the risk of adverse effects from a future ST vaccine may be low. Furthermore, our results suggest that this risk may be reduced or eliminated by basing an ST immunogen on STp or a selectively mutated variant of STh.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Liu M, Ruan X, Zhang C, Lawson SR, Knudsen DE, Nataro JP, Robertson DC, Zhang W. 2011. Heat-labile- and heat-stable-toxoid fusions (LTR192G-STaP13F) of human enterotoxigenic Escherichia coli elicit neutralizing antitoxin antibodies. Infect. Immun. 79:4002–4009. 10.1128/IAI.00165-11 - DOI - PMC - PubMed
-
- Zhang W, Zhang C, Francis DH, Fang Y, Knudsen D, Nataro JP, Robertson DC. 2010. Genetic fusions of heat-labile (LT) and heat-stable (ST) toxoids of porcine enterotoxigenic Escherichia coli elicit neutralizing anti-LT and anti-STa antibodies. Infect. Immun. 78:316–325. 10.1128/IAI.00497-09 - DOI - PMC - PubMed
-
- Zeng W, Azzopardi K, Hocking D, Wong CY, Robevska G, Tauschek M, Robins-Browne RM, Jackson DC. 2012. A totally synthetic lipopeptide-based self-adjuvanting vaccine induces neutralizing antibodies against heat-stable enterotoxin from enterotoxigenic Escherichia coli. Vaccine 30:4800–4806. 10.1016/j.vaccine.2012.05.017 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

