Functional deficiencies of subsarcolemmal mitochondria in the type 2 diabetic human heart
- PMID: 24778174
- PMCID: PMC4080178
- DOI: 10.1152/ajpheart.00845.2013
Functional deficiencies of subsarcolemmal mitochondria in the type 2 diabetic human heart
Abstract
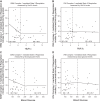
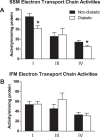
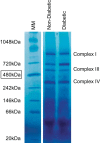
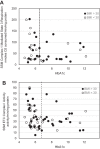
The mitochondrion has been implicated in the development of diabetic cardiomyopathy. Examination of cardiac mitochondria is complicated by the existence of spatially distinct subpopulations including subsarcolemmal (SSM) and interfibrillar (IFM). Dysfunction to cardiac SSM has been reported in murine models of type 2 diabetes mellitus; however, subpopulation-based mitochondrial analyses have not been explored in type 2 diabetic human heart. The goal of this study was to determine the impact of type 2 diabetes mellitus on cardiac mitochondrial function in the human patient. Mitochondrial subpopulations from atrial appendages of patients with and without type 2 diabetes were examined. Complex I- and fatty acid-mediated mitochondrial respiration rates were decreased in diabetic SSM compared with nondiabetic (P ≤ 0.05 for both), with no change in IFM. Electron transport chain (ETC) complexes I and IV activities were decreased in diabetic SSM compared with nondiabetic (P ≤ 0.05 for both), with a concomitant decline in their levels (P ≤ 0.05 for both). Regression analyses comparing comorbidities determined that diabetes mellitus was the primary factor accounting for mitochondrial dysfunction. Linear spline models examining correlative risk for mitochondrial dysfunction indicated that patients with diabetes display the same degree of state 3 and electron transport chain complex I dysfunction in SSM regardless of the extent of glycated hemoglobin (HbA1c) and hyperglycemia. Overall, the results suggest that independent of other pathologies, mitochondrial dysfunction is present in cardiac SSM of patients with type 2 diabetes and the degree of dysfunction is consistent regardless of the extent of elevated HbA1c or blood glucose levels.
Keywords: diabetes mellitus; diabetic cardiomyopathy; mitochondria.
Copyright © 2014 the American Physiological Society.
Figures




References
-
- American Diabetes Association. ADA Diabetes Basics, 2013
-
- Baseler WA, Dabkowski ER, Jagannathan R, Thapa D, Nichols CE, Shepherd DL, Croston TL, Powell M, Razunguzwa TT, Lewis SE, Schnell DM, Hollander JM. Reversal of mitochondrial proteomic loss in type 1 diabetic heart with overexpression of phospholipid hydroperoxide glutathione peroxidase. Am J Physiol Regul Integr Comp Physiol 304: R553–R565, 2013 - PMC - PubMed
-
- Baseler WA, Dabkowski ER, Williamson CL, Croston TL, Thapa D, Powell MJ, Razunguzwa TT, Hollander JM. Proteomic alterations of distinct mitochondrial subpopulations in the type 1 diabetic heart: contribution of protein import dysfunction. Am J Physiol Regul Integr Comp Physiol 300: R186–R200, 2011 - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

