Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis
- PMID: 24778175
- PMCID: PMC4002982
- DOI: 10.1136/bmj.g2688
Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis
Abstract
Objective: To investigate whether discrepancies in trials of use of bone marrow stem cells in patients with heart disease account for the variation in reported effect size in improvement of left ventricular function.
Design: Identification and counting of factual discrepancies in trial reports, and sample size weighted regression against therapeutic effect size. Meta-analysis of trials that provided sufficient information.
Data sources: PubMed and Embase from inception to April 2013.
Eligibility for selecting studies: Randomised controlled trials evaluating the effect of autologous bone marrow stem cells for heart disease on mean left ventricular ejection fraction.
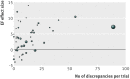
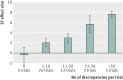
Results: There were over 600 discrepancies in 133 reports from 49 trials. There was a significant association between the number of discrepancies and the reported increment in EF with bone marrow stem cell therapy (Spearman's r=0.4, P=0.005). Trials with no discrepancies were a small minority (five trials) and showed a mean EF effect size of -0.4%. The 24 trials with 1-10 discrepancies showed a mean effect size of 2.1%. The 12 with 11-20 discrepancies showed a mean effect of size 3.0%. The three with 21-30 discrepancies showed a mean effect size of 5.7%. The high discrepancy group, comprising five trials with over 30 discrepancies each, showed a mean effect size of 7.7%.
Conclusions: Avoiding discrepancies is difficult but is important because discrepancy count is related to effect size. The mechanism is unknown but should be explored in the design of future trials because in the five trials without discrepancies the effect of bone marrow stem cell therapy on ejection fraction is zero.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures

Comment in
-
Trials of autologous bone marrow stem cells for heart disease.BMJ. 2014 Apr 29;348:g2750. doi: 10.1136/bmj.g2750. BMJ. 2014. PMID: 24780932 No abstract available.
References
-
- Zimmet H, Porapakkham P, Porapakkham P, Sata Y, Haas SJ, Itescu S, et al. Short- and long-term outcomes of intracoronary and endogenously mobilized bone marrow stem cells in the treatment of ST-segment elevation myocardial infarction: a meta-analysis of randomized control trials. Eur J Heart Fail 2012;14:91-105. - PubMed
-
- Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S, et al. Stem cell treatment for acute myocardial infarction. Cochrane Database Sys Rev 2012;2:CD006536. - PubMed
-
- Francis DP, Mielewczik M, Zargaran D, Cole GD. Autologous stem cell therapy in heart disease: silent discrepancies and scientific and editorial responses. Int J Cardiol 2013;168:3381-403. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
