Endophilin A1 induces different membrane shapes using a conformational switch that is regulated by phosphorylation
- PMID: 24778241
- PMCID: PMC4024918
- DOI: 10.1073/pnas.1402233111
Endophilin A1 induces different membrane shapes using a conformational switch that is regulated by phosphorylation
Abstract
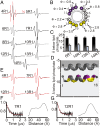
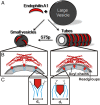
Membrane remodeling is controlled by proteins that can promote the formation of highly curved spherical or cylindrical membranes. How a protein induces these different types of membrane curvature and how cells regulate this process is still unclear. Endophilin A1 is a protein involved in generating endocytotic necks and vesicles during synaptic endocytosis and can transform large vesicles into lipid tubes or small and highly curved vesicles in vitro. By using EM and electron paramagnetic resonance of endophilin A1, we find that tubes are formed by a close interaction with endophilin A1's BIN/amphiphysin/Rvs (BAR) domain and deep insertion of its amphipathic helices. In contrast, vesicles are predominantly stabilized by the shallow insertion of the amphipathic helical wedges with the BAR domain removed from the membrane. By showing that the mechanism of membrane curvature induction is different for vesiculation and tubulation, these data also explain why previous studies arrived at different conclusions with respect to the importance of scaffolding and wedging in the membrane curvature generation of BAR proteins. The Parkinson disease-associated kinase LRRK2 phosphorylates S75 of endophilin A1, a position located in the acyl chain region on tubes and the aqueous environment on vesicles. We find that the phosphomimetic mutation S75D favors vesicle formation by inhibiting this conformational switch, acting to regulate endophilin A1-mediated curvature. As endophilin A1 is part of a protein superfamily, we expect these mechanisms and their regulation by posttranslational modifications to be a general means for controlling different types of membrane curvature in a wide range of processes in vivo.
Keywords: double electron–electron resonance; site-directed spin labeling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


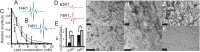
References
-
- McMahon HT, Gallop JL. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature. 2005;438(7068):590–596. - PubMed
-
- Matta S, et al. LRRK2 controls an EndoA phosphorylation cycle in synaptic endocytosis. Neuron. 2012;75(6):1008–1021. - PubMed
-
- Bergmann C, et al. Oligophrenin 1 (OPHN1) gene mutation causes syndromic X-linked mental retardation with epilepsy, rostral ventricular enlargement and cerebellar hypoplasia. Brain. 2003;126(Pt 7):1537–1544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

