Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation
- PMID: 24778247
- PMCID: PMC4024923
- DOI: 10.1073/pnas.1324050111
Large, stratified, and mechanically functional human cartilage grown in vitro by mesenchymal condensation
Abstract
The efforts to grow mechanically functional cartilage from human mesenchymal stem cells have not been successful. We report that clinically sized pieces of human cartilage with physiologic stratification and biomechanics can be grown in vitro by recapitulating some aspects of the developmental process of mesenchymal condensation. By exposure to transforming growth factor-β, mesenchymal stem cells were induced to condense into cellular bodies, undergo chondrogenic differentiation, and form cartilagenous tissue, in a process designed to mimic mesenchymal condensation leading into chondrogenesis. We discovered that the condensed mesenchymal cell bodies (CMBs) formed in vitro set an outer boundary after 5 d of culture, as indicated by the expression of mesenchymal condensation genes and deposition of tenascin. Before setting of boundaries, the CMBs could be fused into homogenous cellular aggregates giving rise to well-differentiated and mechanically functional cartilage. We used the mesenchymal condensation and fusion of CMBs to grow centimeter-sized, anatomically shaped pieces of human articular cartilage over 5 wk of culture. For the first time to our knowledge biomechanical properties of cartilage derived from human mesenchymal cells were comparable to native cartilage, with the Young's modulus of >800 kPa and equilibrium friction coeffcient of <0.3. We also demonstrate that CMBs have capability to form mechanically strong cartilage-cartilage interface in an in vitro cartilage defect model. The CMBs, which acted as "lego-like" blocks of neocartilage, were capable of assembling into human cartilage with physiologic-like structure and mechanical properties.
Keywords: biomimetic; cartilage mechanics; cartilage repair; regenerative medicine; tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

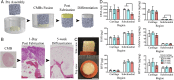

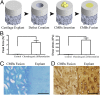
Comment in
-
Regenerative medicine: Dishing up functional human cartilage.Nat Rev Rheumatol. 2014 Jun;10(6):321. doi: 10.1038/nrrheum.2014.81. Epub 2014 May 20. Nat Rev Rheumatol. 2014. PMID: 24846500 No abstract available.
References
-
- Langer RS, Vacanti JP. Tissue engineering: The challenges ahead. Sci Am. 1999;280(4):86–89. - PubMed
-
- Nakao K, et al. The development of a bioengineered organ germ method. Nat Methods. 2007;4(3):227–230. - PubMed
-
- Tew SR, Kwan AP, Hann A, Thomson BM, Archer CW. The reactions of articular cartilage to experimental wounding: Role of apoptosis. Arthritis Rheum. 2000;43(1):215–225. - PubMed
-
- Quinn TM, Allen RG, Schalet BJ, Perumbuli P, Hunziker EB. Matrix and cell injury due to sub-impact loading of adult bovine articular cartilage explants: Effects of strain rate and peak stress. J Orthop Res. 2001;19(2):242–249. - PubMed
-
- Chen CT, Burton-Wurster N, Lust G, Bank RA, Tekoppele JM. Compositional and metabolic changes in damaged cartilage are peak-stress, stress-rate, and loading-duration dependent. J Orthop Res. 1999;17(6):870–879. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

