Capping protein regulatory cycle driven by CARMIL and V-1 may promote actin network assembly at protruding edges
- PMID: 24778263
- PMCID: PMC4024869
- DOI: 10.1073/pnas.1313738111
Capping protein regulatory cycle driven by CARMIL and V-1 may promote actin network assembly at protruding edges
Abstract
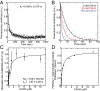
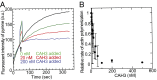
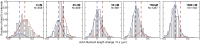
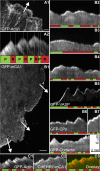
Although capping protein (CP) terminates actin filament elongation, it promotes Arp2/3-dependent actin network assembly and accelerates actin-based motility both in vitro and in vivo. In vitro, capping protein Arp2/3 myosin I linker (CARMIL) antagonizes CP by reducing its affinity for the barbed end and by uncapping CP-capped filaments, whereas the protein V-1/myotrophin sequesters CP in an inactive complex. Previous work showed that CARMIL can readily retrieve CP from the CP:V-1 complex, thereby converting inactive CP into a version with moderate affinity for the barbed end. Here we further clarify the mechanism of this exchange reaction, and we demonstrate that the CP:CARMIL complex created by complex exchange slows the rate of barbed-end elongation by rapidly associating with, and dissociating from, the barbed end. Importantly, the cellular concentrations of V-1 and CP determined here argue that most CP is sequestered by V-1 at steady state in vivo. Finally, we show that CARMIL is recruited to the plasma membrane and only at cell edges undergoing active protrusion. Assuming that CARMIL is active only at this location, our data argue that a large pool of freely diffusing, inactive CP (CP:V-1) feeds, via CARMIL-driven complex exchange, the formation of weak-capping complexes (CP:CARMIL) at the plasma membrane of protruding edges. In vivo, therefore, CARMIL should promote Arp2/3-dependent actin network assembly at the leading edge by promoting barbed-end capping there.
Keywords: VASP; cell migration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112(4):453–465. - PubMed
-
- Chhabra ES, Higgs HN. The many faces of actin: Matching assembly factors with cellular structures. Nat Cell Biol. 2007;9(10):1110–1121. - PubMed
-
- Loisel TP, Boujemaa R, Pantaloni D, Carlier MF. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature. 1999;401(6753):613–616. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

