The clinical approach to small fibre neuropathy and painful channelopathy
- PMID: 24778270
- PMCID: PMC4251302
- DOI: 10.1136/practneurol-2013-000758
The clinical approach to small fibre neuropathy and painful channelopathy
Abstract
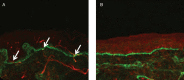
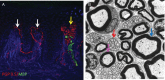
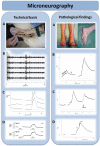
Small fibre neuropathy (SFN) is characterised by structural injury selectively affecting small diameter sensory and/or autonomic axons. The clinical presentation is dominated by pain. SFN complicates a number of common diseases such as diabetes mellitus and is likely to be increasingly encountered. The diagnosis of SFN is demanding as clinical features can be vague and nerve conduction studies normal. New diagnostic techniques, in particular measurement of intraepidermal nerve fibre density, have significantly improved the diagnostic efficiency of SFN. Management is focused on the treatment of the underlying cause and analgesia, as there is no neuroprotective therapy. A recent and significant advance is the finding that a proportion of cases labelled as idiopathic SFN are in fact associated with gain of function mutations of the voltage-gated sodium channels Nav1.7 and Nav1.8 (encoded by the genes SCN9A and SCN10A, respectively). There is a further group of heritable painful conditions in which gain of function mutations in ion channels alter excitability of sensory neurones but do not cause frank axon degeneration; these include mutations in Nav1.7 (causing erythromelalgia and paroxysmal extreme pain disorder) and TRPA1 (resulting in familial episodic pain disorder). These conditions are exceptionally rare but have provided great insight into the nociceptive system as well as yielding potential analgesic drug targets. In patients with no pre-existing risk factor, the investigation of an underlying cause of SFN should be systematic and appropriate for the patient population. In this review, we focus on how to incorporate recent developments in the diagnosis and pathophysiology of SFN into clinical practice.
Keywords: NEUROPATHY; PAIN.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
