Tumoral immune suppression by macrophages expressing fibroblast activation protein-α and heme oxygenase-1
- PMID: 24778275
- PMCID: PMC4007628
- DOI: 10.1158/2326-6066.CIR-13-0150
Tumoral immune suppression by macrophages expressing fibroblast activation protein-α and heme oxygenase-1
Abstract
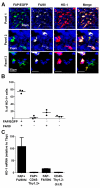
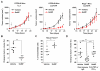
The depletion of tumor stromal cells that are marked by their expression of the membrane protein fibroblast activation protein-α (FAP) overcomes immune suppression and allows an anticancer cell immune response to control tumor growth. In subcutaneous tumors established with immunogenic Lewis lung carcinoma cells expressing ovalbumin (LL2/OVA), the FAP(+) population is comprised of CD45(+) and CD45(-) cells. In the present study, we further characterize the tumoral FAP(+)/CD45(+) population as a minor subpopulation of F4/80(hi)/CCR2(+)/CD206(+) M2 macrophages. Using bone marrow chimeric mice in which the primate diphtheria toxin receptor is restricted either to the FAP(+)/CD45(+) or to the FAP(+)/CD45(-) subset, we demonstrate by conditionally depleting each subset that both independently contribute to the immune-suppressive tumor microenvironment. A basis for the function of the FAP(+)/CD45(+) subset is shown to be the immune inhibitory enzyme, heme oxygenase-1 (HO-1). The FAP(+)/CD45(+) cells are the major tumoral source of HO-1, and an inhibitor of HO-1, Sn mesoporphyrin, causes the same extent of immune-dependent arrest of LL2/OVA tumor growth as does the depletion of these cells. Because this observation of immune suppression by HO-1 expressed by the FAP(+)/CD45(+) stromal cell is replicated in a transplanted model of pancreatic ductal adenocarcinoma, we conclude that pharmacologically targeting this enzyme may improve cancer immunotherapy.
©2013 AACR.
Figures


References
-
- Yu P, Rowley DA, Fu YX, Schreiber H. The role of stroma in immune recognition and destruction of well-established solid tumors. Current Opinion in Immunology. 2006;18:226–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

