Longitudinal requirement for CD4+ T cell help for adenovirus vector-elicited CD8+ T cell responses
- PMID: 24778441
- PMCID: PMC4025612
- DOI: 10.4049/jimmunol.1302806
Longitudinal requirement for CD4+ T cell help for adenovirus vector-elicited CD8+ T cell responses
Abstract
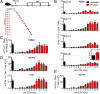
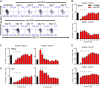
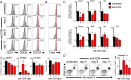
Despite the widespread use of replication-incompetent recombinant adenovirus (Ad) vectors as candidate vaccine platforms, the mechanism by which these vectors elicit CD8(+) T cell responses remains poorly understood. Our data demonstrate that induction and maintenance of CD8(+) T cell responses by Ad vector immunization is longitudinally dependent on CD4(+) T cell help for a prolonged period. Depletion of CD4(+) T cells in wild type mice within the first 8 d following Ad immunization resulted in dramatically reduced induction of Ag-specific CD8(+) T cells, decreased T-bet and eomesodermin expression, impaired KLRG1(+) effector differentiation, and atypical expression of the memory markers CD127, CD27, and CD62L. Moreover, these CD8(+) T cells failed to protect against a lethal recombinant Listeria monocytogenes challenge. Depletion of CD4(+) T cells between weeks 1 and 4 following immunization resulted in increased contraction of memory CD8(+) T cells. These data demonstrate a prolonged temporal requirement for CD4(+) T cell help for vaccine-elicited CD8(+) T cell responses in mice. These findings have important implications in the design of vaccines aimed at eliciting CD8(+) T cell responses and may provide insight into the impaired immunogenicity of vaccines in the context of AIDS and other CD4(+) T cell immune deficiencies.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures






References
-
- Sullivan N. J., Hensley L., Asiedu C., Geisbert T. W., Stanley D., Johnson J., Honko A., Olinger G., Bailey M., Geisbert J. B., et al. 2011. CD8+ cellular immunity mediates rAd5 vaccine protection against Ebola virus infection of nonhuman primates. Nat. Med. 17: 1128–1131 - PubMed
-
- Alexander J., Ward S., Mendy J., Manayani D. J., Farness P., Avanzini J. B., Guenther B., Garduno F., Jow L., Snarsky V., et al. 2012. Pre-clinical evaluation of a replication-competent recombinant adenovirus serotype 4 vaccine expressing influenza H5 hemagglutinin. PLoS ONE 7: e31177. - PMC - PubMed
-
- Colloca S., Barnes E., Folgori A., Ammendola V., Capone S., Cirillo A., Siani L., Naddeo M., Grazioli F., Esposito M. L., et al. 2012. Vaccine vectors derived from a large collection of simian adenoviruses induce potent cellular immunity across multiple species. Sci. Transl. Med. 4: 115ra112 - PMC - PubMed
-
- Xiang Z. Q., Yang Y., Wilson J. M., Ertl H. C. 1996. A replication-defective human adenovirus recombinant serves as a highly efficacious vaccine carrier. Virology 219: 220–227 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

