Review on iron and its importance for human health
- PMID: 24778671
- PMCID: PMC3999603
Review on iron and its importance for human health
Abstract
It is well-known that deficiency or over exposure to various elements has noticeable effects on human health. The effect of an element is determined by several characteristics, including absorption, metabolism, and degree of interaction with physiological processes. Iron is an essential element for almost all living organisms as it participates in a wide variety of metabolic processes, including oxygen transport, deoxyribonucleic acid (DNA) synthesis, and electron transport. However, as iron can form free radicals, its concentration in body tissues must be tightly regulated because in excessive amounts, it can lead to tissue damage. Disorders of iron metabolism are among the most common diseases of humans and encompass a broad spectrum of diseases with diverse clinical manifestations, ranging from anemia to iron overload, and possibly to neurodegenerative diseases. In this review, we discuss the latest progress in studies of iron metabolism and bioavailability, and our current understanding of human iron requirement and consequences and causes of iron deficiency. Finally, we discuss strategies for prevention of iron deficiency.
Keywords: Anemia; human iron requirement; iron bioavailability; iron deficiency; iron metabolism.
Conflict of interest statement
Figures
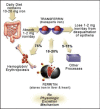

References
-
- Beard JL, Dawson HD. Iron. In: O’Dell BL, Sunde RA, editors. Handbook of Nutritionally Essential Mineral Elements. New York: CRC Press; 1997. pp. 275–334.
-
- Wood RJ, Ronnenberg A. Iron. In: Shils ME, Shike M, Ross AC, Caballero B, Cousins RJ, editors. Modern Nutrition in Health And Disease. 10th ed. Baltimore: Lippincott Williams & Wilkins; 2005. pp. 248–70.
-
- McDowell LR. 2nd ed. Amsterdam: Elsevier Science; 2003. Minerals in Animal And Human Nutrition; p. 660.
-
- Guggenheim KY. Chlorosis: The rise and disappearance of a nutritional disease. J Nutr. 1995;125:1822–5. - PubMed
-
- Yip R, Dallman PR. Iron. In: Ziegler EE, Filer LJ, editors. Present knowledge in nutrition. 7th ed. Washington DC: ILSI Press; 1996. pp. 278–92.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

