Nephrogenic systemic fibrosis risk and liver disease
- PMID: 24778878
- PMCID: PMC3981185
- DOI: 10.1155/2014/679605
Nephrogenic systemic fibrosis risk and liver disease
Abstract
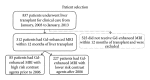
Objective. Evaluate the incidence of nephrogenic systemic fibrosis (NSF) in patients with liver disease in the peritransplant period. Materials and Methods. This IRB approved study retrospectively reviewed patients requiring transplantation for cirrhosis, hepatocellular carcinoma (HCC), or both from 2003 to 2013. Records were reviewed identifying those having gadolinium enhanced MRI within 1 year of posttransplantation to document degree of liver disease, renal disease, and evidence for NSF. Results. Gadolinium-enhanced MRI was performed on 312 of 837 patients, including 23 with severe renal failure (GFR < 30 mL/min/1.73 cm(2)) and 289 with GFR > 30. Two of 23 patients with renal failure developed NSF compared to zero NSF cases in 289 patients with GFR > 30 (0/289; P < 0.003). High dose gadodiamide was used in the two NSF cases. There was no increased incidence of NSF with severe liver disease (1/71) compared to nonsevere liver disease (1/241; P = 0.412). Conclusion. Renal disease is a risk factor for NSF, but in our small sample our evidence suggests liver disease is not an additional risk factor, especially if a low-risk gadolinium agent is used. Noting that not all patients received high-risk gadolinium, a larger study focusing on patients receiving high-risk gadolinium is needed to further evaluate NSF risk in liver disease in the peritransplant period.
Figures
Similar articles
-
Gadolinium-based contrast exposure, nephrogenic systemic fibrosis, and gadolinium detection in tissue.AJR Am J Roentgenol. 2008 Apr;190(4):1060-8. doi: 10.2214/AJR.07.2822. AJR Am J Roentgenol. 2008. PMID: 18356456
-
Nephrogenic systemic fibrosis: center case review.J Magn Reson Imaging. 2007 Nov;26(5):1198-203. doi: 10.1002/jmri.21136. J Magn Reson Imaging. 2007. PMID: 17969162 Review.
-
Nephrogenic systemic fibrosis: incidence, associations, and effect of risk factor assessment--report of 33 cases.Radiology. 2009 Feb;250(2):371-7. doi: 10.1148/radiol.2502080498. Radiology. 2009. PMID: 19188312 Free PMC article.
-
Incidence of nephrogenic systemic fibrosis at two large medical centers.Radiology. 2008 Sep;248(3):807-16. doi: 10.1148/radiol.2483071863. Radiology. 2008. PMID: 18710976
-
Risk factors for developing gadolinium-induced nephrogenic systemic fibrosis.Ann Pharmacother. 2007 Sep;41(9):1481-5. doi: 10.1345/aph.1K295. Epub 2007 Aug 7. Ann Pharmacother. 2007. PMID: 17684032 Review.
Cited by
-
Nephrogenic systemic fibrosis risk after liver magnetic resonance imaging with gadoxetate disodium in patients with moderate to severe renal impairment: results of a prospective, open-label, multicenter study.Invest Radiol. 2015 Jun;50(6):416-22. doi: 10.1097/RLI.0000000000000145. Invest Radiol. 2015. PMID: 25756684 Free PMC article. Clinical Trial.
-
Gadobutrol in Renally Impaired Patients: Results of the GRIP Study.Invest Radiol. 2017 Jan;52(1):55-60. doi: 10.1097/RLI.0000000000000307. Invest Radiol. 2017. PMID: 27529464 Free PMC article. Clinical Trial.
References
-
- Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–1236. - PubMed
-
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. The New England Journal of Medicine. 1996;334(11):693–699. - PubMed
-
- Wong LL. Current status of liver transplantation for hepatocellular cancer. The American Journal of Surgery. 2002;183(3):309–316. - PubMed
-
- United Network for Organ Sharing. Organ distribution: allocation of livers. Policy. 2010;(3.6) http://optn.transplant.hrsa.gov/policiesAndBylaws/policies.asp.
-
- Colli A, Fraquelli M, Casazza G, et al. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. The American Journal of Gastroenterology. 2006;101(3):513–523. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources