Assembly line polyketide synthases: mechanistic insights and unsolved problems
- PMID: 24779441
- PMCID: PMC4020578
- DOI: 10.1021/bi500290t
Assembly line polyketide synthases: mechanistic insights and unsolved problems
Abstract
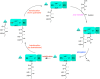
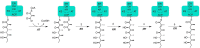
Two hallmarks of assembly line polyketide synthases have motivated an interest in these unusual multienzyme systems, their stereospecificity and their capacity for directional biosynthesis. In this review, we summarize the state of knowledge regarding the mechanistic origins of these two remarkable features, using the 6-deoxyerythronolide B synthase as a prototype. Of the 10 stereocenters in 6-deoxyerythronolide B, the stereochemistry of nine carbon atoms is directly set by ketoreductase domains, which catalyze epimerization and/or diastereospecific reduction reactions. The 10th stereocenter is established by the sequential action of three enzymatic domains. Thus, the problem has been reduced to a challenge in mainstream enzymology, where fundamental gaps remain in our understanding of the structural basis for this exquisite stereochemical control by relatively well-defined active sites. In contrast, testable mechanistic hypotheses for the phenomenon of vectorial biosynthesis are only just beginning to emerge. Starting from an elegant theoretical framework for understanding coupled vectorial processes in biology [Jencks, W. P. (1980) Adv. Enzymol. Relat. Areas Mol. Biol. 51, 75-106], we present a simple model that can explain assembly line polyketide biosynthesis as a coupled vectorial process. Our model, which highlights the important role of domain-domain interactions, not only is consistent with recent observations but also is amenable to further experimental verification and refinement. Ultimately, a definitive view of the coordinated motions within and between polyketide synthase modules will require a combination of structural, kinetic, spectroscopic, and computational tools and could be one of the most exciting frontiers in 21st Century enzymology.
Figures

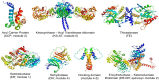

References
-
- Cortes J.; Haydock S.; Roberts G. (1990) An unusually large multifunctional polypeptide in the erythromycin-producing polyketide synthase of Saccharopolyspora erythraea. Nature 348, 176–178. - PubMed
-
- Donadio S.; Staver M.; McAlpine J. (1991) Modular organization of genes required for complex polyketide biosynthesis. Science 252, 675–679. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

