Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy
- PMID: 24779680
- PMCID: PMC4226018
- DOI: 10.1164/rccm.201402-0207OC
Predictors of chronic obstructive pulmonary disease exacerbation reduction in response to daily azithromycin therapy
Abstract
Rationale: Daily azithromycin decreases acute exacerbations of chronic obstructive pulmonary disease (AECOPD), but long-term side effects are unknown.
Objectives: To identify the types of exacerbations most likely to be reduced and clinical subgroups most likely to benefit from azithromycin, 250 mg daily, added to usual care.
Methods: Enrollment criteria included irreversible airflow limitation and AECOPD requiring corticosteroids, emergency department visit, or hospitalization in the prior year or use of supplemental oxygen. Recurrent events and cumulative incidence analyses compared treatment received for AECOPD by randomization group, stratified by subgroups of interest. Cox proportional hazards models estimated treatment effects in subgroups adjusted for age, sex, smoking status, FEV1% predicted, concomitant COPD medications, and oxygen use.
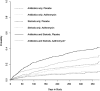
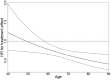
Measurements and main results: Azithromycin was most effective in reducing AECOPD requiring both antibiotic and steroid treatment (n = 1,113; cumulative incidence analysis, P = 0.0002; recurrent events analysis, P = 0.002). No difference in treatment response by sex (P = 0.75), presence of chronic bronchitis (P = 0.19), concomitant inhaled therapy (P = 0.29), or supplemental oxygen use (P = 0.23) was observed. Older age and milder Global Initiative for Chronic Obstructive Lung Disease stage were associated with better treatment response (P = 0.02 and 0.04, respectively). A significant interaction between treatment and current smoking was seen (P = 0.03) and azithromycin did not reduce exacerbations in current smokers (hazard ratio, 0.99; 95% confidence interval, 0.71-1.38; P = 0.95).
Conclusions: Azithromycin is most effective in preventing AECOPD requiring both antibiotic and steroid treatment. Adjusting for confounders, we saw no difference in efficacy by sex, history of chronic bronchitis, oxygen use, or concomitant COPD therapy. Greater efficacy was seen in older patients and milder Global Initiative for Chronic Obstructive Lung Disease stages. We found little evidence of treatment effect among current smokers. Clinical trial registered with www.clinicaltrials.gov (NCT0011986 and NCT00325897).
Trial registration: ClinicalTrials.gov NCT00325897 NCT00011986 NCT00325897.
Keywords: azithromycin; chronic obstructive pulmonary disease; exacerbation; quality of life.
Figures
Comment in
-
[Acute exacerbations of COPD: Prophylactic antibiotics often makes sense].MMW Fortschr Med. 2014 Oct 9;156(17):42. MMW Fortschr Med. 2014. PMID: 25417470 German. No abstract available.
References
-
- Niewoehner DE, Rice K, Cote C, Paulson D, Cooper JA, Jr, Korducki L, Cassino C, Kesten S. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005;143:317–326. - PubMed
-
- Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145:1321–1327. - PubMed
-
- Lawless J. Negative binomial and mixed Poisson regression. Can J Stat. 1987;15:209–225.
-
- Lin DY, Wei LJ, Yang I, Ying Z. Semiparametric regression for the mean and rate functions of recurrent events. J R Statist Soc B. 2000;62:711–730.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 HL074416/HL/NHLBI NIH HHS/United States
- U10 HL074424/HL/NHLBI NIH HHS/United States
- U10 HL074428/HL/NHLBI NIH HHS/United States
- U10 HL074439/HL/NHLBI NIH HHS/United States
- R01 NR013377/NR/NINR NIH HHS/United States
- U10 HL074408/HL/NHLBI NIH HHS/United States
- U10 HL074422/HL/NHLBI NIH HHS/United States
- U10 HL074407/HL/NHLBI NIH HHS/United States
- U10 HL074418/HL/NHLBI NIH HHS/United States
- U10 HL074409/HL/NHLBI NIH HHS/United States
- U10 HL074431/HL/NHLBI NIH HHS/United States
- U10 HL074441/HL/NHLBI NIH HHS/United States
- U10HL074424/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

