Cisplatin intrastrand adducts sensitize DNA to base damage by hydrated electrons
- PMID: 24779712
- PMCID: PMC4623755
- DOI: 10.1021/jp5014913
Cisplatin intrastrand adducts sensitize DNA to base damage by hydrated electrons
Abstract
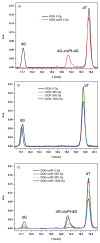
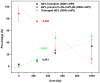
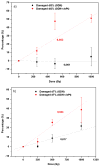
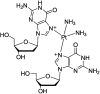
The oligonucleotide TTTTTGTGTTT with or without a cisplatin adduct was reacted with hydrated electrons generated by ionizing radiation. Hydroxyl radicals were quenched with ethylenediaminetetraacetic acid (EDTA), and the solutions were bubbled with wet nitrogen to eliminate oxygen, a scavenger of hydrated electrons. Prior to irradiation, the structure of the initial cisplatin adduct was identified by mass spectrometry as G-cisplatin-G. Radiation damage to DNA bases was quantified by high-performance liquid chromatography (HPLC), after enzymatic digestion of the TTTTTGTGTTT-cisplatin complex to deoxyribonucleosides. The masses of the platinum adducts following digestion and separation by HPLC were measured by mass spectrometry. Our results demonstrate that hydrated electrons induce damage to thymines as well as detachment of the cisplatin moiety from both guanines in the oligonucleotide. This detachment regenerates both unmodified guanine and damaged guanine, in equimolar amounts. At 1000 Gy, a net average of 2.5 thymines and 1 guanine are damaged for each platinum lost from the oligonucleotide. Given the extensive base damage that occurs for each cisplatin adduct lost, it is clear that, prior to undergoing detachment, these adducts must catalyze several cycles of reactions of hydrated electrons with DNA bases. It is likely that a single reaction leads to the loss of the cisplatin adduct and the damage observed on the guanine base; however, the damage to the thymine bases must require the continued presence of the cisplatin adduct, acting as a catalyst. To our knowledge, this is the first time that platinum-DNA adducts have been shown to have catalytic activity. We propose two pathways for the interaction of hydrated electrons with TTTTTGTGTTT-cisplatin: (1) the hydrated electron is initially captured by a thymine base and transferred by base to base electron hopping to the guanine site, where the cisplatin moiety detaches from the oligonucleotide via dissociative electron attachment, and (2) the hydrated electron interacts directly with the platinum-guanine adduct and induces detachment of the cisplatin moiety via dissociative electron attachment. Although the precise mechanism remains to be elucidated, our results provide important insights into the radiosensitization of DNA by cisplatin.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
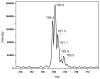
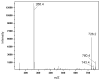
References
-
- Samant S, Kumar P, Wan J, Hanchett C, Vieira F, Murry T, Wong FSH, Robbins KT. Concomitant Radiation Therapy and Targeted Cisplatin Chemotherapy for the Treatment of Advanced Pyriform Sinus Carcinoma: Disease Control and Preservation of Organ Function. Head Neck. 1999;21:595–601. - PubMed
-
- Boscolo-Rizzo P, Gava A, Marchiori C, Baggio V, da Mosto MC. Functional Organ Preservation in Patients with Locoregionally Advanced Head and Neck Squamous Cell Carcinoma Treated by Platinum-Based Multidrug Induction Chemotherapy and Concurrent Chemoradiotherapy. Ann Oncol. 2011;22:1894–1901. - PubMed
-
- Peters WA, III, Liu PY, Barrett RJ, II, Stock RJ, Monk BJ, Berek JS, Souhami L, Grigsby P, Gordon W, Alberts DS. Concurrent Chemotherapy and Pelvic Radiation Therapy Compared with Pelvic Radiation Therapy Alone as Adjuvant Therapy after Radical Surgery in High-Risk Early-Stage Cancer of the Cervix. J Clin Oncol. 2000;18:1606–1613. - PubMed
-
- Choy H, Mac Rae RM. Chemoradiation in Cancer Therapy. Humana Press; Totowa, NJ: 2003.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

