The contribution of ketone bodies to basal and activity-dependent neuronal oxidation in vivo
- PMID: 24780902
- PMCID: PMC4083391
- DOI: 10.1038/jcbfm.2014.77
The contribution of ketone bodies to basal and activity-dependent neuronal oxidation in vivo
Abstract
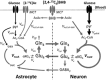
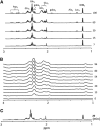
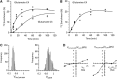
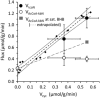
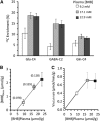
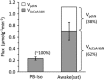
The capacity of ketone bodies to replace glucose in support of neuronal function is unresolved. Here, we determined the contributions of glucose and ketone bodies to neocortical oxidative metabolism over a large range of brain activity in rats fasted 36 hours and infused intravenously with [2,4-(13)C₂]-D-β-hydroxybutyrate (BHB). Three animal groups and conditions were studied: awake ex vivo, pentobarbital-induced isoelectricity ex vivo, and halothane-anesthetized in vivo, the latter data reanalyzed from a recent study. Rates of neuronal acetyl-CoA oxidation from ketone bodies (V(acCoA-kbN)) and pyruvate (V(pdhN)), and the glutamate-glutamine cycle (V(cyc)) were determined by metabolic modeling of (13)C label trapped in major brain amino acid pools. V(acCoA-kbN) increased gradually with increasing activity, as compared with the steeper change in tricarboxylic acid (TCA) cycle rate (V(tcaN)), supporting a decreasing percentage of neuronal ketone oxidation: ∼100% (isoelectricity), 56% (halothane anesthesia), 36% (awake) with the BHB plasma levels achieved in our experiments (6 to 13 mM). In awake animals ketone oxidation reached saturation for blood levels >17 mM, accounting for 62% of neuronal substrate oxidation, the remainder (38%) provided by glucose. We conclude that ketone bodies present at sufficient concentration to saturate metabolism provides full support of basal (housekeeping) energy needs and up to approximately half of the activity-dependent oxidative needs of neurons.
Figures
References
-
- Lopes-Cardozo M, Larsson OM, Schousboe A. Acetoacetate and glucose as lipid precursors and energy substrates in primary cultures of astrocytes and neurons from mouse cerebral cortex. J Neurochem. 1986;46:773–778. - PubMed
-
- Künnecke B, Cerdan S, Seelig J. Cerebral metabolism of [1,2-13C2]glucose and [U-13C4]3-hydroxybutyrate in rat brain as detected by 13C NMR spectroscopy. NMR Biomed. 1993;6:264–277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

