Pavlovian conditioning and cross-sensitization studies raise challenges to the hypothesis that overeating is an addictive behavior
- PMID: 24780921
- PMCID: PMC4012290
- DOI: 10.1038/tp.2014.28
Pavlovian conditioning and cross-sensitization studies raise challenges to the hypothesis that overeating is an addictive behavior
Abstract
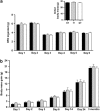
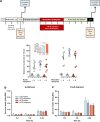
Elevated glucocorticoid levels and sign tracking (ST) in Pavlovian conditioning are potential biomarkers of compulsive behaviors such as addiction. As overeating is sometimes viewed as a form of addictive behavior, we hypothesized that murine Pavlovian sign trackers would have a greater propensity to overeat and develop obesity. Using a food reward in the classical conditioning paradigm, we show that ST behavior is a robust conditioned response but not a predictor of eating and growth trajectories in mice, thus challenging the view that the development of obesity and drug addiction depend on identical mechanisms. This interpretation was supported by experiments which showed that overweight mice do not display cross-sensitization to an addictive drug (morphine), and conversely, that overweight morphine-sensitized animals do not overconsume a highly rewarding food. Although the rewarding/motivational effects of both food and drugs of abuse are mediated by similar neurochemical mechanisms, obesity and drug addiction represent a summation of other dysfunctional input and output pathways that lead to the emergence of two distinct disorders, each of which would deserve a specific pharmacotherapeutic approach.
Figures



Similar articles
-
Post-trial low dose apomorphine prevents the development of morphine sensitization.Behav Brain Res. 2020 Feb 17;380:112398. doi: 10.1016/j.bbr.2019.112398. Epub 2019 Nov 29. Behav Brain Res. 2020. PMID: 31790782
-
Addictive potential of modafinil and cross-sensitization with cocaine: a pre-clinical study.Addict Biol. 2011 Oct;16(4):565-79. doi: 10.1111/j.1369-1600.2011.00341.x. Epub 2011 Jul 25. Addict Biol. 2011. PMID: 21790900
-
Lesion of olfactory epithelium attenuates expression of morphine-induced behavioral sensitization and reinstatement of drug-primed conditioned place preference in mice.Pharmacol Biochem Behav. 2013 Jan;103(3):526-34. doi: 10.1016/j.pbb.2012.10.009. Epub 2012 Oct 24. Pharmacol Biochem Behav. 2013. PMID: 23103203
-
Mapping sign-tracking and goal-tracking onto human behaviors.Neurosci Biobehav Rev. 2020 Apr;111:84-94. doi: 10.1016/j.neubiorev.2020.01.018. Epub 2020 Jan 20. Neurosci Biobehav Rev. 2020. PMID: 31972203 Free PMC article. Review.
-
Overlaps in the nosology of substance abuse and overeating: the translational implications of "food addiction".Curr Drug Abuse Rev. 2011 Sep;4(3):133-9. doi: 10.2174/1874473711104030133. Curr Drug Abuse Rev. 2011. PMID: 21999687 Review.
Cited by
-
Is eating behavior manipulated by the gastrointestinal microbiota? Evolutionary pressures and potential mechanisms.Bioessays. 2014 Oct;36(10):940-9. doi: 10.1002/bies.201400071. Epub 2014 Aug 8. Bioessays. 2014. PMID: 25103109 Free PMC article. Review.
-
Male Goal-Tracker and Sign-Tracker Rats Do Not Differ in Neuroendocrine or Behavioral Measures of Stress Reactivity.eNeuro. 2021 May 6;8(3):ENEURO.0384-20.2021. doi: 10.1523/ENEURO.0384-20.2021. Print 2021 May-Jun. eNeuro. 2021. PMID: 33731330 Free PMC article.
-
Reward components of feeding behavior are preserved during mouse aging.Front Aging Neurosci. 2014 Sep 16;6:242. doi: 10.3389/fnagi.2014.00242. eCollection 2014. Front Aging Neurosci. 2014. PMID: 25278876 Free PMC article.
-
Food and addiction among the ageing population.Ageing Res Rev. 2015 Mar;20:79-85. doi: 10.1016/j.arr.2014.10.002. Epub 2014 Oct 17. Ageing Res Rev. 2015. PMID: 25449527 Free PMC article. Review.
-
Altered motivation masks appetitive learning potential of obese mice.Front Behav Neurosci. 2014 Oct 30;8:377. doi: 10.3389/fnbeh.2014.00377. eCollection 2014. Front Behav Neurosci. 2014. PMID: 25400563 Free PMC article.
References
-
- Scott KM, McGee MA, Wells JE, Oakley Browne MA. Obesity and mental disorders in the adult general population. J Psychosom Res. 2008;64:97–105. - PubMed
-
- Allison DB, Newcomer JW, Dunn AL, Blumenthal JA, Fabricatore AN, Daumit GL, et al. Obesity among those with mental disorders: a National Institute of Mental Health meeting report. Am J Prev Med. 2009;36:341–350. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

