Cockayne syndrome protein A is a transcription factor of RNA polymerase I and stimulates ribosomal biogenesis and growth
- PMID: 24781187
- PMCID: PMC4111694
- DOI: 10.4161/cc.29018
Cockayne syndrome protein A is a transcription factor of RNA polymerase I and stimulates ribosomal biogenesis and growth
Abstract
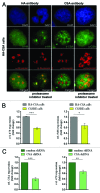
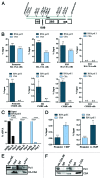
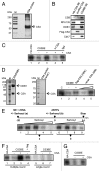
Mutations in the Cockayne syndrome A (CSA) protein account for 20% of Cockayne syndrome (CS) cases, a childhood disorder of premature aging and early death. Hitherto, CSA has exclusively been described as DNA repair factor of the transcription-coupled branch of nucleotide excision repair. Here we show a novel function of CSA as transcription factor of RNA polymerase I in the nucleolus. Knockdown of CSA reduces pre-rRNA synthesis by RNA polymerase I. CSA associates with RNA polymerase I and the active fraction of the rDNA and stimulates re-initiation of rDNA transcription by recruiting the Cockayne syndrome proteins TFIIH and CSB. Moreover, compared with CSA deficient parental CS cells, CSA transfected CS cells reveal significantly more rRNA with induced growth and enhanced global translation. A previously unknown global dysregulation of ribosomal biogenesis most likely contributes to the reduced growth and premature aging of CS patients.
Keywords: Cockayne syndrome; DNA repair; RNA polymerase I transcription; growth; premature aging; ribosomopathy.
Figures

References
-
- Nance MA, Berry SA. Cockayne syndrome: review of 140 cases. Am J Med Genet. 1992;42:68–84. - PubMed
-
- Itoh T, Ono T, Yamaizumi M. A new UV-sensitive syndrome not belonging to any complementation groups of xeroderma pigmentosum or Cockayne syndrome: siblings showing biochemical characteristics of Cockayne syndrome without typical clinical manifestations. Mutat Res. 1994;314:233–48. doi: 10.1016/0921-8777(94)90068-X. - DOI - PubMed
-
- Horibata K, Iwamoto Y, Kuraoka I, Jaspers NG, Kurimasa A, Oshimura M, Ichihashi M, Tanaka K. Complete absence of Cockayne syndrome group B gene product gives rise to UV-sensitive syndrome but not Cockayne syndrome. Proc Natl Acad Sci U S A. 2004;101:15410–5. doi: 10.1073/pnas.0404587101. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
